TOPIC 1: OXYGEN
Oxygen exists in air to an extent of 21% by volume (or 23% by weight). It is the most abundant element on earth, accounting for ½ the total mass of the earth’s crust. Oxygen is mainly found in combined states as oxides, hydroxides, silicates, sulphates, carbonates, water, etc. Its ease of combination with other elements to form compounds shows that oxygen is a very reactive element.
Preparation and Properties of Oxygen
Oxygen can be prepared in the laboratory from either hydrogen peroxide solution or potassium chlorate salt.
A Sample of Oxygen Gas in the Laboratory
Prepare a sample of oxygen gas in the laboratory
(i) Laboratory preparation of oxygen from hydrogen peroxide solution
The most common method for the preparation of oxygen in the laboratory is by decomposition of hydrogen peroxide solution. The gas is prepared by catalysing the decomposition of hydrogen peroxide with manganese (IV) oxide. At room temperature hydrogen peroxide decomposes (breaks down) very slowly. It decomposes to water and oxygen.
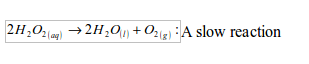
To speed up the decomposition process, and hence collect substantial amount of oxygen gas within a short time, black manganese (IV) oxide is added as a catalyst.
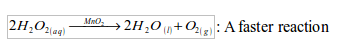
A catalyst is a substance that, although present in small quantities, will alter the rate of a chemical reaction but will remain chemically unchanged at the end of the reaction.
Preparation method
Hydrogen peroxide (20 vol.) is added drop by drop to manganese (IV) oxide, which catalyses the decomposition of the peroxide. Oxygen is collected over water as shown in figure bellow. The gas is collected by downward displacement of water because it is only slightly soluble in water.

Apparatus for laboratory preparation of oxygen from hydrogen peroxide solution
(ii) Laboratory preparation of oxygen from potassium chlorate
Oxygen can also be prepared by thermal decomposition of potassium chlorate. When this compound is heated, it decomposes slowly into potassium chloride and oxygen:

Preparation method
A grinded mixture of potassium chlorate and manganese (IV) oxide, at a ratio of 4:1, is placed in hard glass tube and fitted up as shown in figure bellow. The mixture is then heated and oxygen gas is readily given off. The gas is collected over water. Oxygen has almost the same density as air, so it cannot be collected by the upward displacement of air. It is possible to collect it by downward displacement of water as shown in the figure because it is only slightly soluble in water.
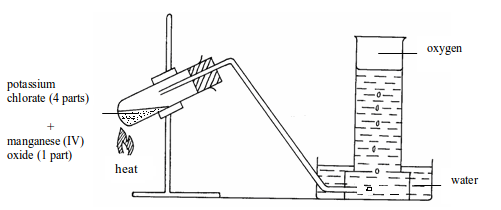
Apparatus for laboratory preparation of oxygen from potassium chlorate
Test for oxygen
Oxygen rekindles a glowing splint of wood. No gases behave like this except dinitrogen oxide, NO2, from which oxygen can be distinguished by the following properties:
1. Oxygen has no smell but dinitrogen oxide has a sweet, sickly smell.
2. When heated with nitrogen monoxide, oxygen produces brown fumes of nitrogen dioxide.
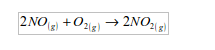
Dinitrogen oxide has no effect on nitrogen monoxide.
Simple Experiments to Demonstrate Properties of Oxygen Gas
Perform simple experiments to demonstrate properties of oxygen gas
1. Action of oxygen on metals
The manner in which oxygen reacts with metals is summarized in the list below.

Reaction with specific metals
Sodium
When burnt in excess of oxygen, sodium burns with an intense yellow flame to give sodium peroxide.
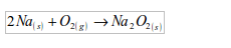
The product is a yellow solid which dissolves in water to give an alkaline solution.
Calcium
The metal burns in air with a red flame giving a white solid of calcium oxide:
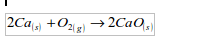
Magnesium
Magnesium burns with a brilliant white flame, leaving a white ash of magnesium oxide:
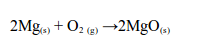
Iron
Iron burns in air with a shower of sparks leaving a brown-black solid of triiron tetraoxide:
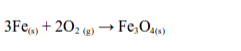
Copper
Copper burns in a stream of oxygen to give a black solid of copper (II) oxide:
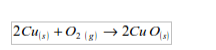
In general, metals react with oxygen to form basic oxides.
Action of oxygen on non-metals
Carbon
Red-hot carbon combines vigorously with oxygen to form carbon dioxide, giving no residue:
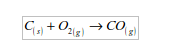
Sulphur
Sulphur burns with a blue flame giving misty white fumes of sulphur dioxide:
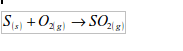
Phosphorus
Phosphorus bursts into flame in air or oxygen, without being heated (that is why it is stored under water). A white solid, phosphorus pentoxide is formed.
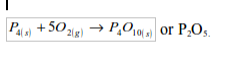
Properties of Oxygen
Explain properties of oxygen
Physical properties
- It is a clear, colourless gas with no smell.
- It is a neutral gas (it is neither basic nor acidic in character)
- It is slightly soluble in water (100 cm3of water at room temperature dissolves about 4 cm3 of oxygen).
- It has almost the same density as water although slightly denser than air. 5. It boils at -183ºC and freezes at -218ºC.
Chemical properties
- Oxygen supports combustion
- It is a very strong oxidizing agent.
- Oxygen is very reactive. It reacts vigorously with a great many metals and non-metals to form basic and acidic oxides respectively. Metal + Oxygen gives metallic oxide (most of these are basic in character). Non-metals + Oxygen gives non–metallic oxide (most of these are acidic in character).
Uses of Oxygen
Uses of Oxygen in Daily Life
List uses of oxygen in daily life
includes
- The oxygen in the air and that dissolved in water and soil is used by all respiring organisms. Also all types of burning need oxygen.
- It is used in the oxyacetylene (oxygen–ethyne) flame for welding and cutting steel.
- It is extensively used for removing impurities from pig iron in order to produce steel. Oxygen is blown into molten iron to remove impurities such as carbon or phosphorus, which are expelled in the form of gases, i.e. their oxides.
- Oxygen is used as an aid to breathing in hospitals, high altitude climbing or flying, and in deep sea diving.
- Liquid oxygen is used in the burning of fuels such as kerosene, hydrogen and hydrazine used in various types of rockets.
- It is used in the L-D process for making steel.
Relationship between Some Uses of Oxygen to its Properties
Relate some uses of oxygen to its properties
There is relationship between uses of hydrogen and its properties. For example, oxygen is used as an aid to breathing in hospitals and at extreme altitudes because it supports life, and for combustion because it supports burning. Likewise, due to its highly reactive nature, oxygen is used for removal of impurities, welding, in the L-D process for making steel, and in burning of fuels in rockets.
TOPIC 2: HYDROGEN
Hydrogen is the lightest of all the elements. There is very little hydrogen in the earth's atmosphere. Hydrogen is so light that its molecules are not held by the earth's gravity and they diffuse into space. Overall, it is the most common element in the universe. It is probable that is forms about 90% of the total mass of the universe. It is believed that the sun composes almost of hydrogen and helium. Hydrogen occurs naturally in air as hydrogen gas. It also occurs in combined state in water, acids, petroleum, and natural gas and in almost all organic substances (proteins, carbohydrates, fats, etc.).
Preparation and Properties of Hydrogen
The Preparation of Hydrogen Gas in a Laboratory
Explain the preparation of hydrogen gas in a laboratory
Hydrogen is most commonly prepared in the laboratory by the action of dilute mineral acids on certain metals. The most convenient way to prepare hydrogen in the laboratory is by addition of dilute hydrochloric acid on zinc granules. Zinc and hydrochloric acid are chosen because they produce the gas at a steady rate.
The gas may be collected by downward displacement of water. But when the gas is required free from moisture it is passed through water to remove first, any hydrogen chloride gas and then through concentrated sulphuric acid to remove moisture before being collected by upward delivery. The gas is prepared by upward delivery method because it is lighter than air and is soluble in water.
Method of preparation
Set up the apparatus as shown in figure bellow. Into a flat-bottomed flask, put some pieces of zinc and add dilute hydrochloric acid by means of a thistle funnel. There is effervescence, and a gas is given off which is collected over water. Zinc chloride, which is formed, dissolves to form zinc chloride solution.
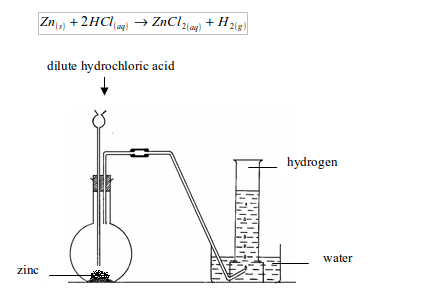
Preparation of hydrogen by the action of dilute hydrochloric acid on zinc metal
Test for hydrogen
A mixture of hydrogen and air explodes with a 'pop' sound when a flame is applied.
The Properties of Hydrogen
Explain the properties of hydrogen
Physical properties
Includes
- It is a colourless, tasteless and odourless gas.
- It is almost insoluble in water (2 volumes of hydrogen gas dissolve in 100 volumes of water at 8ºC).
- It is the lightest of all gases. It is about 20 times lighter than air (one litre of hydrogen at 0ºC and 760 mmHgpressure weighs 0.0899 grams)
- It condenses at -254ºC to a colourless liquid (and liquid hydrogen freezes at -259 ºC to form colourless crystals).
- It is neutral to litmus. 6. It does not support combustion.
Chemical properties
1. It combines easily with other chemical substances at high temperatures. For example, it combines with oxygen to form water. A mixture of the two gases will not react at room temperature. At higher temperatures, or when a flame is applied, the mixture will explode. When hydrogen and oxygen explode, the product is water.
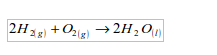
Water is just the common name for the substance "hydrogen oxide".
2. Hydrogen acts as a reducing agent, by removing oxygen from some compounds. For example, copper (II) oxide is reduced to copper by heating it in a stream of hydrogen. The hydrogen is oxidized to water.
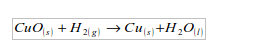
3. It is neither acidic nor basic, so it a neutral gas.
4. A mixture of hydrogen and oxygen explodes when lit.
An experiment on reduction of copper (II) oxide (CuO) using hydrogen
Aim: To investigate the effect of hydrogen on copper (II) oxide
Procedure
- Put about 5 g of copper (II) oxide in a Pyrex test tube and set up the apparatus as shown in figure bellow. Observe and note the colour of copper (II) oxide before the start of the experiment. What colour is it?
- By means of a thistle funnel, add hydrochloric acid in a bottle containing zinc metal to generate hydrogen gas. Pass the gas through a U-tube containing a solid drying agent, calcium chloride.
- Place a dry cobalt (II) chloride paper near the mouth of a test tube as shown in figure bellow.
- Allow the hydrogen gas to pass through the apparatus for some time in order to displace all the air before lighting it.
- Heat the copper (II) oxide strongly until no further changes in colour of the cobalt (II) chloride paper takes place. You may repeat the experiment using lead (II) oxide and compare the results.
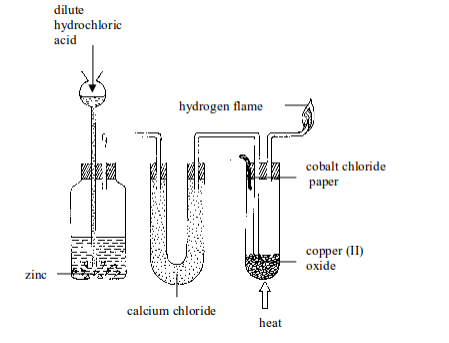
Reduction of copper (II) oxide with hydrogen gas
Questions
- What happens to the copper (II) oxide during the experiment?
- (a) What happens to cobalt (II) chloride paper? (b)Why is it used? (c) What other substance can serve the same purpose as cobalt (II) chloride paper?
- Enough time should be allowed for all the air in the test tube to be replaced by hydrogen before lighting the gas. What is bad about lighting a mixture of air and hydrogen?
- What do you think can cause the size of the hydrogen flame to deteriorate?
- (a) What element did hydrogen take from the copper (II) oxide? (b) Can hydrogen take the same element from any metal oxide?
Answers
1. Black copper (II) oxide is reduced by hydrogen to brown copper metal.
2. (a) Cobalt (II) chloride paper changes its colour from blue to pink.
(b) The paper is used to indicate that water has been formed in the reaction between copper (II) oxide and hydrogen. This water turns the colour of the paper from blue to pink.
(c) The other substance that can be used instead of cobalt (II) chloride paper is white anhydrous copper (II) sulphate, which turns blue when in contact with water.
3. Enough time should be allowed for hydrogen to replace the air in the test-tube because a mixture of hydrogen and oxygen in the tube is explosive when lit.
4. The size of the hydrogen flame deteriorates with time as hydrogen supply grows smaller following complete displacement of hydrogen of the hydrochloric acid with zinc. Deterioration can also be caused by use of excess copper (II) oxide or strong heating, meaning that most hydrogen is used in the reduction of the oxide.
5. (a) The element taken by hydrogen from copper (II) oxide is oxygen. In this experiment, hydrogen reduces copper (II) oxide to copper, while hydrogen itself is oxidized to water:
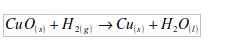
(b) No. Hydrogen can only reduce those metals that are below it in the electrochemical (activity) series.
Uses of Hydrogen
Uses of Hydrogen Gas
State uses of hydrogen gas
1. It is used in the manufacture of ammonia by the Haber process, which is based on the direct combination of hydrogen and nitrogen.
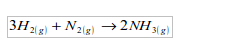
2. It is used in the hardening of vegetable oils to make margarine.
3. It was formerly used for inflating balloons and air ships. But hydrogen is inflammable and many accidents occurred. Its use has been replaced by helium (another gas occurring in air). Nowadays, hydrogen is used by meteorologists to fill weather balloons, which carry weather instruments that record information on various elements of weather in the upper atmosphere.
4. It is used to prepare water gas, which is used as a fuel for space rockets. When hydrogen contained in water gas is burned in air, it produces extremely high heat energy that is used to power rocket engines.
5. It is used in welding by the atomic hydrogen torch. The complete combustion between hydrogen and oxygen is a highly exothermic reaction and can produce an oxy-hydrogen flame that has a temperature of nearly 2000ºC, and is therefore useful in the welding and cutting of metals. However, the explosive nature of the combustion of hydrogen with oxygen makes the use of oxy-hydrogen flame less favourable than the oxyacetylene flame.
6. It is used in the synthesis of hydrochloric acid. In this case, hydrogen combines directly with chlorine to form hydrogen chloride gas.
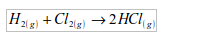
The hydrogen chloride gas is then dissolved in water to form hydrochloric acid.
7. It is used in the manufacture of methanol (wood spirit). In this process hydrogen combines directly with carbon monoxide.
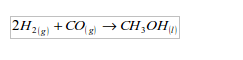
TOPIC 3: WATER
Occurrence and Nature of Water
The Occurrence and Nature of Water
Describe the occurrence and nature of water
Water is the most abundant liquid in nature. It is a compound of hydrogen and oxygen. It occurs on land as seas, oceans, rivers, springs, wells, etc. It also occurs in the atmosphere as rain, water vapour, clouds, etc. Water is the essential constituent of animal and plant life. Without water, no life could exist on earth. All living things need water to survive. About 60% of the human body by mass is made of water. A human being needs to drink about 2 litres of water per day to replace the water lost from the body via sweat, urine, breath, faeces, etc. If you did not replace this by eating and drinking, you would die in a matter of days.
Water is more important than food. A human being can survive without food for many weeks, but will die in a few days without water. So without water, no life can be sustained.
Water is the main constituent of the earth's surface. 70% of the earth's surface is covered by water. The remaining 30% is covered by land.
Types of water
There are four kinds of natural water namely, rain water, spring and well water, river water, and lake and sea water. Natural water is never pure. Water from difference natural sources contains substances dissolved in it.
Rain water
This is naturally distilled water. It is almost pure and it contains only gases and dust dissolved from the air. If the dissolved gases are acidic, e.g. sulphur dioxide, carbon dioxide or nitrogen dioxide, they may form "acid rain". In heavily industrialized countries where emission of these gases is very great, acid rains have been experienced. Rain water in non-industrial areas is fairly pure. It is safe to drink though it is tasteless. The taste in water is due to dissolved substances in it.
Spring and well water
When the rain falls, some water sinks into the ground to form ground water. This water percolates down the earth until it meets layers of impervious or impermeable (non-porous) rocks, which stop it from percolating or seeping any further. The ground water may reach the earth's surface as a spring. When a whole deep enough is dug to reach the ground water, a well results. Spring or well water is supposed to be clean, although it contains dissolved substances. As water passes through the earth, it is naturally filtered.
River water
River water contains dissolved and suspended solid materials. The water in some rivers is very muddy or sandy depending on the nature of the land from which the river originates and on which it flows. Most of the water we drink or use at home and industries is from rivers. To make the river water fit for use, all the substances dissolved and suspended in it must be removed or filtered.
Lake and sea water
Lakes and seas receive water from rivers. River water contains dissolved salts. As it flows through the land, some of its water evaporates into the air. When it reaches the sea or lake, more water still evaporates. As a result, sea and lake water will necessarily contain vast quantities of dissolved substances. Sea water contains about 3.6% by mass of the dissolved solids. Most of the dissolved solids compose largely of sodium chloride that can be obtained from sea water in large quantities. Three quarters of the ocean salts is sodium chloride (common salt).
The Water Cycle
Describe the water cycle
Water is always on move, travelling a never-ending, cyclical journey between the earth and the sky. This journey is referred to as the water cycle or hydrological cycle. The water cycle describes the continuous movement of water on, above and below the surface of the earth. During its movement, water is continuously reused and recycled. It also changes its physical state or form (liquid, vapour, and ice) at various stages in the water cycle. Figure 3.1 is a diagrammatic representation of the water cycle. It shows how the water moves around the earth's environment, changing its form through the process of evaporation, transpiration (loss of water from plants), condensation and precipitation (rainfall, snow, hail, fog, smog, etc.) Stages of the water cycle are described below:
- Heat from the sun causes water to evaporate from exposed water bodies such as oceans, seas, lakes, rivers dams, etc. This causes huge amounts of water vapour to float (laden) in the air. The vapour rises up. In the cooler upper parts of the atmosphere, the vapour cools and condenses to form tiny water droplets. The droplets form clouds.
- The clouds are drifted by wind. They cool further, and the droplets join to form larger drops of water which fall down as rain due to gravitation pull. On the other hand, if the air is very cold, they fall as hail, sleet or snow. The whole process is called precipitation.
- Some rain water soaks, and reappears as springs. Some flows over the ground as streams. The springs and streams feed rivers. The rivers flow to the ocean, sea or lake. The whole cycle starts again.
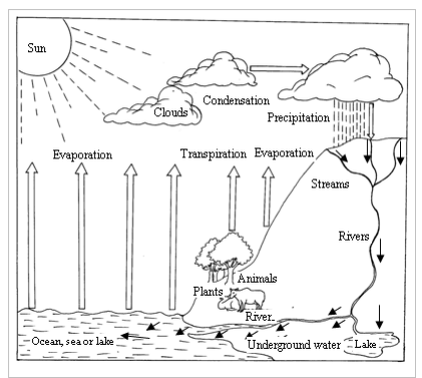
The water cycle
Water Cycle and Environmental Conservation
Relate water cycle to environmental conservation
Everyone understands why it is so important to keep our water clean. The fresh water that is available for use by people, plants and animals must be clean and safe.
Sometimes human carelessness pollutes the water system, loading harmful and unhealthy substances into the system at a rate that exceeds its natural restorative capabilities. When harmful substances are discarded (disposed off; dumped) into the environment, they may very well end up as part of the water cycle. An example of these acts may happen when untreated municipal and industrial wastes are directed into the water bodies such as rivers, lakes and seas. These substances are toxic and may harm human, marine, animal and plant life.
When chemicals are released into the air, they might well return to the earth with rain and snow or by simply settling. For example in industrial areas, sulphur dioxide dissolves in water from the clouds and with oxygen from the atmosphere to form sulphuric acid.
Sulphur dioxide + water + oxygen gives sulphuric acid = “acid rain”
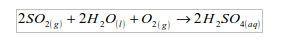
This then falls as "acid rain". The acid rain washes salts from the top soil. Acidic water and metal salts run into the lakes or rivers. The introduction of these new substances consequently increases the acidity and concentration of metal salts in the lake, river or stream. As a result, fish and other marine life die.
Nitrogen oxides, NOx, can also cause acid rain. When nitrogen dioxide gas reacts with water and oxygen in the atmosphere, the result is a weak solution of nitric acid.
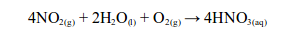
Carbon dioxide also reacts with water in the atmosphere to form a weak carbonic acid (rain water).
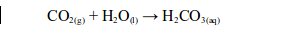
Pure water has a pH of 7.0. Normal rain is slightly acidic because of the carbon dioxide gas dissolved into it. It has a pH of about 5.5.
It has been confirmed that carbon dioxide (CO2), sulphur dioxide (SO2) and nitrogen oxides (NOx) are the primary causes of acid rain.
When harmful substances are dumped on land or buried in the ground, they might well find their way into ground water or surface water. These substances contaminate the water, which may be someone's or some community's drinking water.
Water plays an important role in the conservation of the environment and in determining human settlement and development. It also governs plant and animal distribution. Animals and plants, as components of the environment, are mainly concentrated in water or in areas where water is found.
Plant roots bind the soil particles together, making the soil compact and less susceptible to erosion. However, vegetation will only grow and flourish on land that receives sufficient rainfall. This is possible only if the water cycle is properly maintained by conserving natural forests and planting more trees to attract rainfall. So it is obvious that there is a strong relationship between rainfall (as a crucial stage of the water cycle) and the vegetation and soil (as components of the environment).
We use water from the lakes, rivers, wells or springs to irrigate crop and non-crop plants. So, when we distort the water cycle in some way or the other we may not have enough rainfall to fill up rivers or springs from which we obtain the water we use to conserve our environment (vegetation).
Properly watered soils support more plants. We all know that plants absorb carbon dioxide from the atmosphere, therefore, helping to purify the air naturally. In addition, plants produce oxygen gas, which is needed by all living organisms. If there is not enough rainfall, most plants will die, hence resulting to excessive accumulation of carbon dioxide, which may rise to toxic levels.
Excessive carbon dioxide in the atmosphere leads to intense heating of the earth's surface, a phenomenon described as global warming. The consequence of global warming include encroachment and extension of desert and arid lands, prolonged droughts, changes in rainfall patterns, etc.
These few facts show that there is a strong relationship and correlation between environmental conservation and the water cycle. Environmental degradation can lead to serious and irreparable aftermath to the water cycle.
Properties of Water
Simple Experiments on Physical and Chemical Properties of Water
Perfom simple experiments on physical and chemical properties of water
Activity 1
Perfom simple experiments on physical and chemical properties of water.
Properties of Water
Explain properties of water
Physical properties
Includes
- Extremely pure water is colourless, odourless and tasteless. The colour, taste or odours in water are due to dissolved impurities of organic and inorganic nature.
- Pure water is a very poor conductor of heat and electricity. However, water containing some dissolved inorganic impurities may conduct appreciably.
- Pure water freezes at 0ºC.
- Pure water boils at 100ºC at a pressure of 760 mmHg; and pure water will boil away completely with no change in temperature. Its melting point and boiling point are abnormally high due to hydrogen bonding.
- It is the only substance that occurs naturally in all the three states of matter – solid, liquid and gas.
- Water, as compared to other liquids, dissolves almost all substances, though in varying degrees of solubility. For this reason, water is usually called the universal solvent.
- It has a high surface tension than other liquids.
- It has a high specific heat index, which means that it can absorb a lot of heat before getting hot.
- It is miscible with many liquids, for example ethanol.
- The maximum density of pure water is 1 g cm-3 at 4ºC. When water is cooled gradually, it reaches its maximum density at 4 centigrade. The actual change from water to ice takes place at 0ºC.
- Pure water is neutral to litmus and has a pH of 7.0.
- Water expands when it freezes. Most substances contract when they change from liquid to solid state. Water is one of the very few substances that expand when they freeze. This behaviour is called anomalous expansion of water. Ice is therefore much less dense than water. The water molecules in the ice crystals are further apart from each other than in liquid water.
Chemical properties
Action of heat
Water is extremely stable to heat. A stable compound does not decompose easily by heating. It requires a very high temperature to decompose water. Water decomposes lightly at 2500ºC. It approaches complete decomposition at 5000ºC
Reaction with metals
The state in which water reacts with metals depends on the position of a metal in the electrochemical series as shown below:

It can be seen that water attacks metals differently depending on the metal’s position in the activity series. This is called the chemical activity series of metals.
Potassium
Potassium is vigorously attacked with cold water, producing hydrogen gas. The reaction of water with potassium is very violent and the hydrogen produced catches fire spontaneously with a lilac flame. The colour is due to the burning of small quantities of potassium vapour.
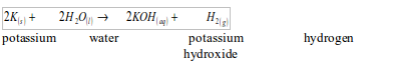
Sodium
The reaction of sodium with water is vigorous but the hydrogen liberated does not catch fire. Sodium reacts with cold water to produce hydrogen gas, which is detected by effervescence as the gas is liberated. If a flame is applied, it burns with a yellow flame (the yellow colour is from sodium).
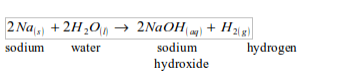
Calcium
Calcium reacts with water relatively slowly compared to sodium and potassium. The gas (hydrogen) given off explodes if mixed with air, and if a flame applied.
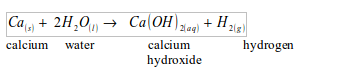
Magnesium
Magnesium reacts with steam to liberate hydrogen and magnesium oxide.

Zinc
If zinc is heated to redness in a current of steam, hydrogen is liberated.
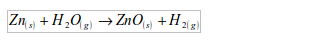
Iron
Iron does not react with cold water, but readily reacts with excess steam at red heat.
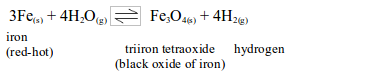
The above reaction can be made to proceed in the reverse direction by passing excess of hydrogen over heated triiron tetraoxide.
Reaction with non-metals
Carbon
Red-hot carbon reacts with steam at 1000ºC to give a mixture of carbon monoxide and hydrogen, known as water gas.
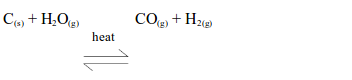
Red-hot carbon reacts with steam at 1000ºC to give a mixture of carbon monoxide and hydrogen, known as water gas.
Chlorine reacts with water to form a mixture of two acids.

Reaction with oxides
1. Water reacts with the oxides of most reactive metals to form hydroxides:
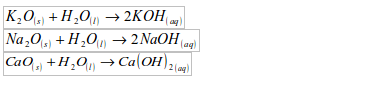
2. Water reacts with the oxides of some non–metals to form acids:

Formation of hydrates
Water combines with many salts to form hydrates. Different salt hydrates have different number of molecules of water of crystallization. The following are some examples:
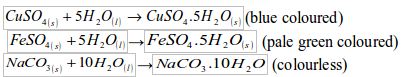
Synthesis of water
Water is a compound of hydrogen and oxygen. Its formula is H2O. You could make it in the laboratory by burning a jet of hydrogen in air. The reaction is fast and dangerous:
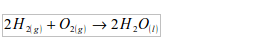
Hence, in the synthesis of water, hydrogen has to be prepared and then reacted with the oxygen of the air to give water. The apparatus used for water synthesis is as shown in figure 3.2 Hydrogen is produced by the reaction between zinc and cold dilute sulphuric acid
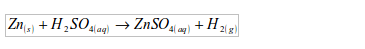
But the hydrogen to be used for synthesis of water has to be absolutely dry. This is achieved by passing it through anhydrous calcium chloride. It is then allowed to pass through the jet. When the hydrogen has displaced all the air in the apparatus, the gas is lit at the jet. The water forms as a gas. The gas condenses to liquid on an ice-cold tube. The burning hydrogen reacts with the oxygen of the air as given by the equation below:
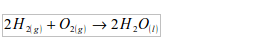
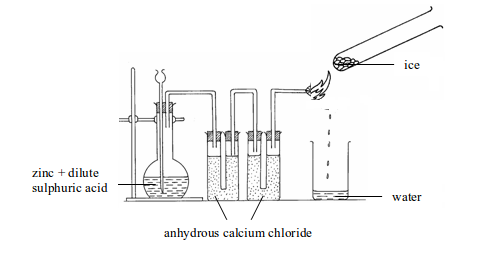
Physical tests for water
1. Water can be recognized by its action of turning white anhydrous copper (II) sulphate to blue.

The test, however, confirms the presence of water and not the absence of everything else except water. For example, a dilute sulphuric acid would turn anhydrous copper (II) sulphate from white to blue. That is why this test is called a physical test as opposed to a chemical test for water.
2. The presence of water can also be shown by the use of cobalt chloride paper. This is a filter paper impregnated with cobalt (II) chloride. The paper is blue in colour. The blue paper turns pink when in contact with water.
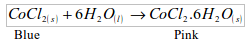
A chemical test for water
The two tests above only confirm the presence of water but do not indicate the purity of the water. Now, how can we test if an unknown colourless liquid contains water or if it is pure water? The presence of water will do the following:
- Will turn anhydrous copper (II) sulphate from white to blue.
- Will turn anhydrous cobalt (II) chloride from blue to pink.
To find out if a liquid is pure water, its boiling point or its freezing point must be measured. Pure water boils at exactly 100ºC and freezes at 0ºC at pressure of one atmosphere (760 mmHg). This is a chemical test for water.
Treatment and Purification of Water
Processes of Domestic Water Treatment and Purification
Perform processes of domestic water treatment and purification
Water for domestic use is chiefly obtained from rivers, springs and wells; and sometimes from lakes and seas. However, lake and sea waters may be to too salty for drinking or washing and hence not normally used for such purposes. But for some countries, the sea is a major source of drinking water. However, this water must be desalinized (have its salt removed) and purified before being used for drinking. The process is very expensive. It involves an expenditure of big sums of money. It is only practised in developed countries.
River and spring water must be boiled and filtered before drinking. At homes, water is normally boiled in big pans, cooled down, and then filtered by using a white, sterile and clean piece of cloth. The cloth is tied around the mouth of the container as shown in figure 3.3(a). As water is poured through the cloth, the particles in it are filtered off. The clean water is then poured in clay pots or plastic buckets and placed in a cool place, or put in a refrigerator to cool down ready for drinking.
Alternatively, boiled water can be filtered using a funnel as shown in figure (b) bellow, but you must ensure the gravel, sand and cotton wool used are thoroughly sterile. Sterilization can be achieved by soaking the gravel and sand in hot boiled water for quite some time.
The gravel traps any large floating substances. The coarse sand prevents small particles from passing through. The fine sand ensures even the small suspended particles do not pass through, while the cotton wool filters the very tiny particles.
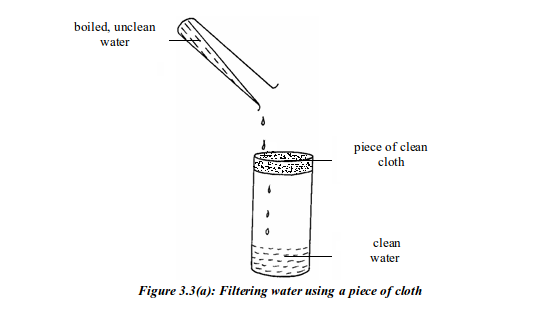
(a): Filtering water using a piece of cloth
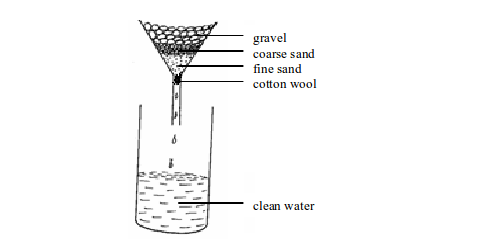
(b): Filtering water using a funnel
At home, water can also be purified with chemical purifiers. These chemicals are in liquid or tablet form. To purify water, a recommended amount of the purifier is added to a specific amount of water in a container. The water is shaken or stirred well. Then it is left to settle for at least 20 minutes before it can be safe for drinking and other domestic uses. To get the clearest water, it is advisable to filter the water thoroughly before adding the purifier. The commonest and most widely used purifiers are the waterguard and aquaguard.
In developed countries, commercial filters may be used to purify water at home. These filters contain charcoal or ceramic element that purifies the water as it passes through the filter.
The Processes of Urban Water Treatment
Describe the processes of urban water treatment
We obtain our water supply from surface water (for example, rivers, lakes and reservoirs) and ground water (for example, underground aquifers and lakes). Water from these sources is never completely pure, particularly if it is drawn from a river. The water may contain:
- bacteria – most are harmless, but some can cause diseases.
- dissolved substances – for example, calcium and magnesium compounds dissolved from rocks; and gases from the air.
- solid substances and debris – particles of mud, sand, grit, twigs, dead plants and perhaps tins and rags that people have dumped.
All these impurities are gathered by water as it passes through different parts of land as rivers or streams. Before water is safe to drink, the bacteria and solid substances must be removed.
Different towns and regions of the world apply different methods of water treatment. The more sophisticated and expensive methods are used by rich nations such as the UK and USA. Some steps in water treatment, however, are basic and used by all. They include the following:
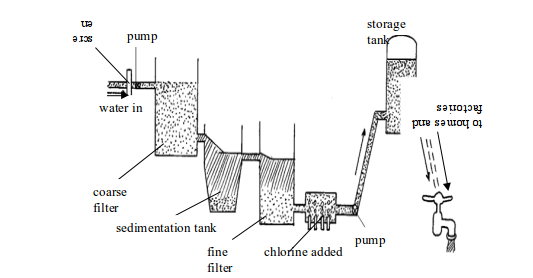
Urban water treatment and purification
1. After water has been pumped through the screen to get rid of the larger bits of rubbish, it is pumped through a coarse filter which traps larger particles of solid. The filter could be beds of gravel and fine sand or anthracite.
2. In older purification plants, it may go to a sedimentation tank where chemicals are added to make smaller particles stick together. Then they sink to the bottom of the tank. Many chemicals could be used but the basic ones are the following:
(i) copper sulphate to remove algae;
(ii) sodium carbonate for softening; and
(iii) Aluminium sulphate in the form of potash alum, K2SO4.AL2(SO4)3.24H2O and slaked lime,Ca(OH)2are added for coagulating and precipitating all the suspended earthy material (clay matter). Bacteria and other microorganisms are captured by the coagulated mud, and precipitated. Sometimes instead of potash alum, iron (III) alum, (NH4)2.Fe(SO4)3.24H2Ocan be used.
The two chemicals (potash alum and slaked lime) react to form aluminium hydroxide and calcium sulphate:
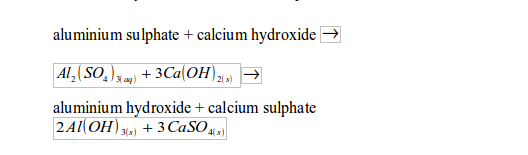
The aluminium hydroxide is bulky and sticky. Therefore, bacteria, microorganisms and small particles can stick to it and get precipitated. The calcium sulphate is by far denser than water. Both the solid products (aluminium hydroxide, plus organic and inorganic particles stuck on it, and calcium sulphate) sink to the bottom of the tank. The whole process is called sedimentation.
3. Water from the sedimentation tank is passed through a fine filter. The filter could be made of layers of sand, gravel or carbon granules with thousands of tiny pores. The carbon removes coloured matter, odours (tastes) and noxious smells from the water. Filtration beds are expensive to install and require considerable labour to maintain.
4. After filtering, the water is chlorinated and may be aerated. Chlorine is added to kill harmful bacteria. Chlorine is such a useful disinfectant that it is used in swimming pools to kill bacteria. In aeration, water is pumped through fountains and sprout into the air. Aeration kills many dangerous aquatic bacteria. In some countries and regions, water is fluorinated by adding sodium fluoride to the water supply to help prevent tooth decay. Finally, the water is pumped to storage tanks, and then to homes and factories.
Importance of water treatment and purification
It is very important that community water supply be well treated and purified. There are several reasons for this practice. The following are some of the reasons:
- To kill harmful and disease-causing microorganisms such as bacteria, fungi, actinomycetes, amoeba, salmonella, etc.
- To remove toxic substances dissolved in water
- To remove solid substances and debris from the water such as tins, lags, plant remains, sand, algae, spirogyra, etc.
- To remove suspended earthy material (clay matter)
- To remove odour and unpleasant smells caused by different contaminants dissolved in water.
- To remove water hardness - sodium carbonate is added in water to remove both temporary and permanent hardness in water to make the water soft. Soft water forms lather easily with soap as compared to hard water which forms scum instead. This means that soft water requires less soap to form enough lather than hard water does. Therefore, soft water saves soap and hence money that could have been spent to purchase extra soap for washing.
- The sodium fluoride added to water in some areas helps to fight tooth decay.
Uses of Water
Uses of Water
State uses of water
Water is one of the most vital natural resources for all life on earth. The availability and quantity of water have always played an important part in determining not only where people can live, but also their quality of life. Even though there always has been plenty of fresh water on earth, water has not always been available when and where it is needed, nor is it always suitable for all uses. Water must be considered as a finite resource that has limits and boundaries to its availability and sustainability for use.
Where water supply is limited, conflicts may result between and among the various uses. The balance between supply and demand for water is a delicate one. The availability of usable water has and will continue to dictate where and to what extent development will occur. Water must be in sufficient supply for an area to develop, and an area cannot continue to develop if water demand far exceeds supply.
Water has numerous uses in life. The following are some of the uses of water:
- Biological use: Water is essential to life. Most of the reactions in animals and plants take place in solutions in water. Plants absorb minerals from the soil in solution form. Animals and plants are found near or in areas where water can be found.
- Domestic use: Domestic water use is probably the most important daily use of water for most people. It includes water that is used in the home every day including water for normal household purposes such as washing clothes and dishes, drinking, bathing, food preparation, flushing toilets, and watering lawns and gardens, etc.
- Industrial use: Water is a valuable resource to the nation’s industries for such purposes as processing, cleaning, transportation, dilution, and cooling in manufacturing industries. Major water-using industries include cloth, steel, chemical, paper, and petroleum refining. Industries often reuse the same water repeatedly for more than one purpose. Water is used as a solvent in many industrial processes. It is also used for cooling certain parts of machines.
- Irrigation: Water is artificially applied to farm, orchard pasture, and horticultural crops, as well as leaching of salts from the crop root zone in sodic soils. Non-agricultural activities include self-supplied water to irrigate public and private flower gardens, loans, football pitches, etc. Crop production in areas that receive little rainfall per year can be achieved through the practice of irrigation. Water for irrigation purposes can be drawn from rivers, lakes, swamps and even from seas.
- Water as a solvent: Water is regarded as a universal solvent. It dissolves almost all substances. For this reason, it is used for dissolution of chemicals ranging from poisonous chemicals used in agriculture to non-poisonous chemicals used in hospitals, laboratories, research stations and for other general purposes.
- Cooling and heating: Due to its high specific heat capacity, water is used as a coolant for cooling automobile engines and other machines. Hot water is used during winter for heating homes in temperate countries. In higher plants, evaporation causes a cooling effect and therefore helps to cool plant organs. During hot weather, some animals tend to wallow in water in order to cool their bodies either through evaporation or by water itself.
- Habitat: Water is a habitat for fish and all aquatic animals and plants.
- Livestock use: This includes water for stock animals, feedlots, dairies, fish farms and other non-farm animals. In arid regions of Tanzania, the Government has constructed dams to supply water to cattle, and for some domestic uses.
- Mining: Water is used in mines for extraction of naturally occurring minerals: solids, such as coal and ores; liquids, such as crude petroleum; and gases, such as natural gas. This includes quarrying, milling (such as crushing, screening, washing, and flotation), and other operations as part of mining activity.
- Generation of electricity:Hydroelectric power is generated by river water. Fast-moving river water (especially in waterfalls and cataracts) is used to turn turbines to generate hydroelectricity that is supplied to homes, industries, towns, etc. Most of the electricity we use at home is generated by this means. Only a small portion is generated through other means.
- Navigation and recreation: People, goods and services can be transported via water bodies like rivers, lakes and oceans by using vessels such as boats, dhows, canoes and ships. Water is also used for sports such as swimming, canoeing, fishing, yachting, water skiing, and many other sports carried out on, in and under the water.
The Solubility of Different Substances in Water and Organic Solvents
Compare the solubility of different substances in water and organic solvents
Water is a very good solvent for many ionic substances. There are few substances, which do not dissolve in water to some extent. Even when you drink a glass of water, you are also drinking a little of the glass as well. The amount is very small indeed, but for certain experiments ordinary glass vessels cannot be used as containers for water because of this solvent effect. Water is the commonest solvent in use, but other liquids, are also important. The other solvents are generally organic liquids such as ethanol, propanone, trichloroethane, etc. These organic solvents are also important because they will often dissolve substances that do not dissolve in water. The following table shows an example of substances that dissolve in water.
Substances soluble and insoluble in water
| Soluble compounds | Insoluble compounds |
| 1 All common sodium, potassium and ammonium salts | |
| 2. All common nitrates of metals | |
| 3. All common chlorides except………………….. | silver, mercury (I) and lead chloride |
| 4. All common sulphates except………………….. | lead, barium and calcium sulphates |
| 5. Sodium, potassium, and ammonium carbonates… | but other common carbonates are insoluble |
| 6. Sodium, potassium and ammonium hydroxides… | but other common hydroxides are insoluble. |
When salt is added to water and the mixture stirred, the salt dissolves. The product formed is termed as a solution. The solid that dissolves is known as a solute and the liquid (water) in which a solute dissolves is a solvent.
We can continue to add more salt and stir until no more salt dissolves. At this point, the water has dissolved the maximum amount of salt possible. The amount of salt dissolved denotes the maximum amount of salt which can normally be held in solution.
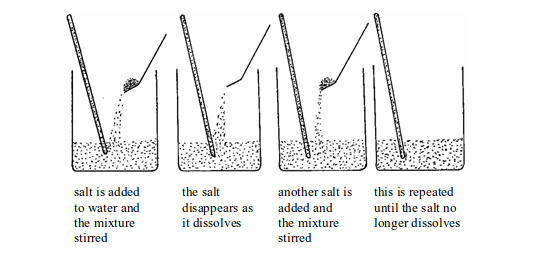
Adding a salt to water
The solution made is called saturated solution. The amount of the salt that has dissolved is called the solubility of the salt in water. The solubility of a substance is usually expressed as the mass of the substance dissolved in 100g of water. Solubility is sometimes expressed in moles of solute per dm3 of solution at that temperature.
To give a quantitative meaning to solubility, it is necessary to fix the amount of the solvent used and to state the temperature at which dissolution occurs. The amount of solvent is usually fixed at 100g. For example, the solubility of sugar (sucrose) at 20ºC is 240g in 100g of water. What is the maximum weight of sugar that will dissolve at 20ºC in a cup containing 350g of water? A saturated solution of a solute at a particular temperature is the one which will not dissolve any more of the solute at that temperature.
The solubility of a solute in water at a given temperature is the maximum amount of it that will dissolve in 100g of water at that temperature.
Dissolving a solid in water
Generally, the solubility of a solute increases with increase in temperature. However, there are a few exceptions e.g. the solubility of calcium hydroxide decreases with increase in temperature. Sugar dissolves very slowly in water at room temperature (20ºC). Stirring helps to make sugar dissolve more quickly. But if you keep on adding sugar to the water even with continuous stirring, eventually no more sugar will dissolve. Extra sugar sinks to the bottom. The solution is saturated.
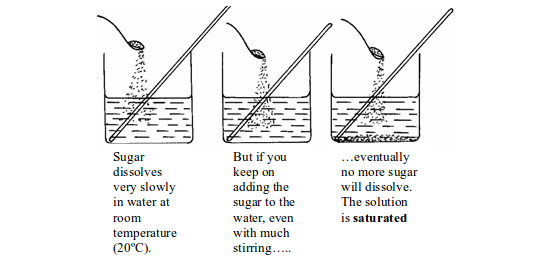
Dissolving a solid in water at room temperature
Now let us look at what happens when you heat the sugar solution. If you heat the solution up to 20ºC there is still undissolved sugar at the bottom of the beaker. Increasing the temperature to 50ºC makes some sugar dissolve but there is still some left. But if the temperature is raised up to 80ºC all the sugar dissolves. You might even be able to dissolve more sugar!
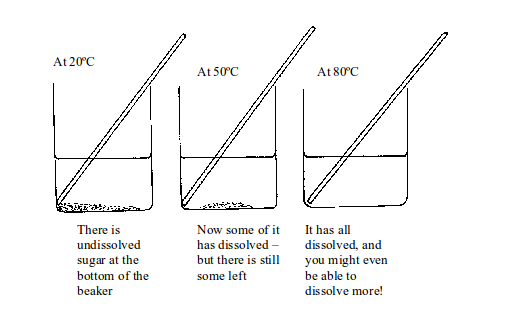
Dissolving a solid in water at higher temperatures
Therefore, sugar is more soluble in hot than in cold water. In fact, this is usually the case with soluble solids. If a solid is soluble in a liquid, it usually gets more soluble as the temperature rises.
Solubility of different substances in different solvents
The solubility of a substance depends on the following factors:
1. The type of solvent used: Iodine is slightly soluble in water. Only 0.3g will dissolve in 100g of water at 20ºC. However, it is much more soluble in cyclohexane (organic solvent). 2.8g of iodine dissolve in 100g of cyclohexane at 20ºC
2. The particles in it: Let us consider the dissolution of sodium chloride in water. When dissolved in water, the salt dissolves to form Na+ and Cl- ions. If sodium chloride is added to water, the Na+ ions will be attracted to the slightly negatively charged oxygen atoms of the water molecules whereas Cl-ions will be attracted to the slightly positively charged hydrogen atoms of the water.
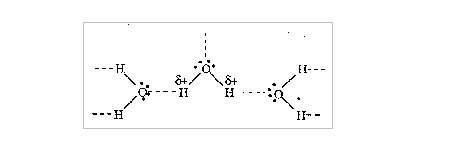
3. The temperature of the solvent: As we saw early, the temperature affects the solubility of substances, particularly solids. The higher the temperature the higher is the solubility.
If you shake some cyclohexane with a solution of iodine in water, almost all iodine leaves the water and moves into cyclohexane layer. So, cyclohexane is much better than water at separating iodine particles from each other. The iodine particles are more attracted to cyclohexane than they are to water. So, the solubility of each substance is different. Look at these examples:
| Compound | Mass (g) dissolving in 100g of water at 25ºC |
| Silver nitrate | 241.3 |
| Calcium nitrate | 102.1 |
| Magnesium chloride | 53.0 |
| Potassium nitrate | 37.9 |
| Potassium sulphate | 12.0 |
| Calcium hydroxide | 0.113 |
| Calcium carbonate | 0.0013 |
| Silver chloride | 0.0002 |
As you can see, one compound of a metal may be slightly soluble while another is almost soluble (compare silver nitrate and silver chloride). It depends on particles.
Measuring the solubility of a solid in water
Let us take potassium sulphate as our example. This is what to do:
- Put a weighed amount (say 2g) of potassium sulphate in a test tube. Add a little water from a measuring cylinder.
- Heat the test tube gently until the water is hot but not boiling. Add more water if necessary until the solid is just dissolved.
- Let the solution cool while stirring it with a thermometer. Note the temperature at which the first crystals form.

Measuring the solubility of a solid in water
Now look again at step 3. If you add a little more water, heat the solution again to make sure all the crystals have dissolved, and then let it cool, you will be able to find the solubility at a lower temperature. You can repeat this for a range of temperatures.
Calculating solubility
Since you know the mass of solute and the volume of water you used, you can work out the solubility as shown in the calculation below:
Example 1
2 grams of potassium sulphate were dissolved in 12.5 cm3 of water. On cooling, the first crystals appeared at 60ºC. What is the solubility of potassium sulphate in water at 60ºC?
Solution
12.5 cm3 of water weighs 12.5g. Also, remember that solubility is measured by 100g of water. If 2g of the salt dissolved in 12.5g of water, then the amount of the salt in 100g of water.

Therefore, the solubility of potassium sulphate in water at 60ºC is 16 grams.
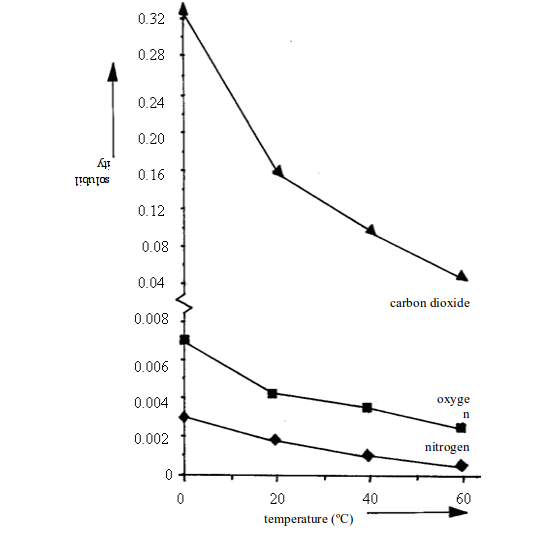
Solubility of gases
Solid solutes usually get more soluble in water as the temperature rises. The opposite is true for gases. Table 3.3 shows the solubility of different gases in water at different temperatures.
Solubility of different gases in water
| Gas | Solubility (cm3 per 100cm3 of water) at..... | |||
| 0ºC | 20ºC | 40ºC | 60ºC | |
| Oxygen Carbon dioxide Sulphur dioxide Hydrogen chloride | 4.8171798050500 | 3.392.3425047400 | 2.556.6217044500 | 1.936.0-42000 |
Look at carbon dioxide. It is quite soluble in water at room temperature (20ºC). But when it is pumped into soft drinks under pressure, a lot more dissolves. Then when you open the bottle, it fizzes out of solution.
Look at hydrogen chloride. At room temperature, it is over 14000 times more soluble than oxygen.
Generally, the solubility of gases changes with temperature and pressure. It decreases with temperature and increases with pressure.
Solubility curves
The solubility of a particular solid in water can be measured over a range of temperatures up to 100ºC. The maximum mass of solid that will dissolve in 100g of water is found at each temperature. The values at each temperature can then be plotted to give a solubility curve. A curve that shows how the solubility of a substance changes with temperature is what we call a solubility curve.
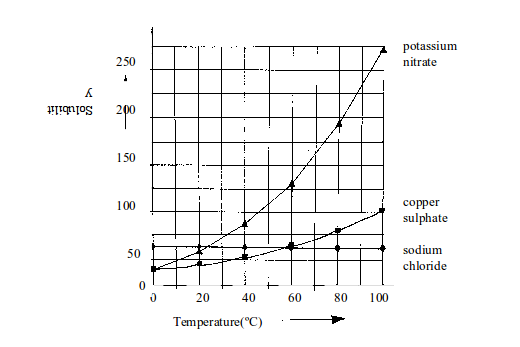
Table bellow shows the solubility of some salts in water at different temperatures.
Solubility of some salts in water
| Temperature in ºC | Solubility in g of salt per 100g of water | ||
| Sodium chloride | Copper (II) sulphate | Potassium nitrate | |
| 10 | 38 | 18 | 20 |
| 20 | 38 | 20 | 30 |
| 30 | 38 | 24 | 44 |
| 40 | 38.5 | 28 | 60 |
| 50 | 38.5 | 34 | 80 |
| 60 | 39 | 42 | 104 |
| 70 | 39 | 50 | 152 |
For most substances, solubility in water increases with increase in temperature. Table above shows the solubility of some salts in water at different temperatures.
When the values for each salt shown on the table are represented on a graph paper, different solubility curves result.
Look at the values in table above again. On a graph paper, use the same set of axes to plot solubility (vertical axis) against temperature (horizontal axis). Draw a smooth best-fit curve for each salt.
- Which of the salts is the most soluble at 15ºC?
- Which of the three salts is the most soluble at 55ºC?
- At which temperature do sodium chloride and potassium nitrate have the same solubility?
The curves in figure bellow show how the solubility of different salts changes with temperature. You can see that the solubility of most solids increases with increase in temperature. The increase for sodium chloride is very small and almost negligible. The increase for the other salts is as shown in the graph.
Solubility curves for three solids in water (solubility measured in grams of solid per 100g of water)
For gases, the solubility decreases with increase in temperature. This means that decreasing the temperature will increase the solubility of gases. Figure 3.10 shows the solubility curves for some common gases. Compare these curves with those for solids in figure above.
The solubility of three gases from the air in water (solubility measured in grams of gas per 100g of water)
Using solubility curves
Data can be obtained from the solubility curves in various ways. For example, look at figure above.
(a) What mass of potassium nitrate dissolves in 100g of water at
- 40ºC and
- 50ºC?
From the graph:
- At 50ºC, 137.5g of potassium nitrate dissolve in 100g of water.
- At 40ºC, 62.5g of potassium nitrate dissolve in 100g of water.
(b) What mass of potassium nitrate will crystallize out when a saturated solution in 100g of water is cooled from 50ºC to 40ºC?
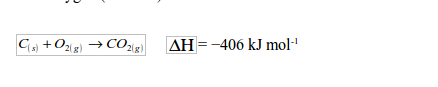

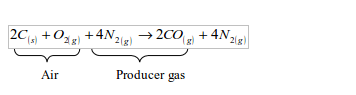


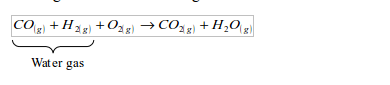
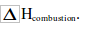

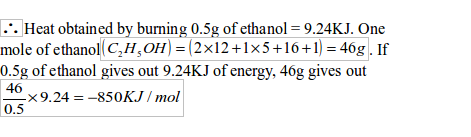
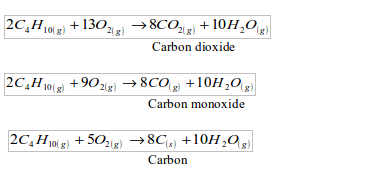
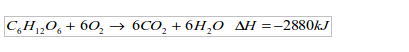
TOPIC 5: ATOMIC STRUCTURE
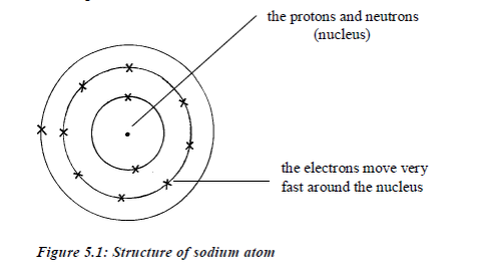
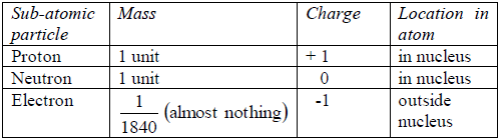
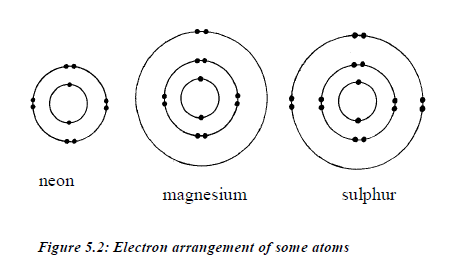
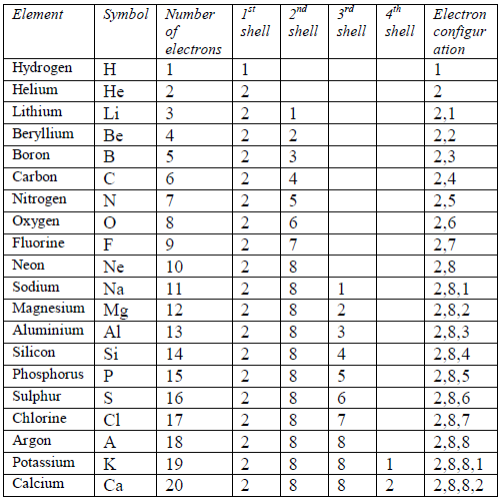
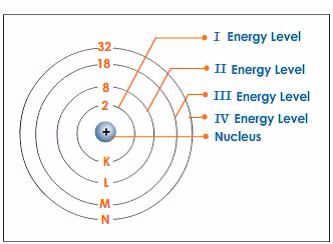
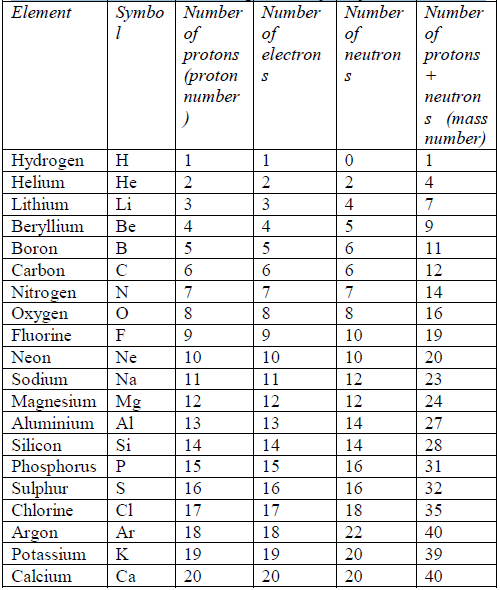
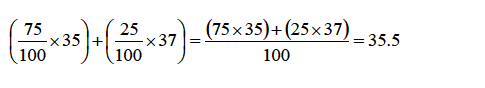
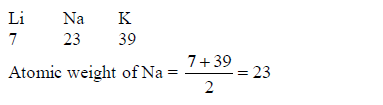


TOPIC 4: FUEL AND ENERGY
A fuel is a substance that can be combusted or burnt to release energy as a byproduct. The energy can be in the form of heat, light, electricity, sound etc. This energy can be harnessed to power machines or used for other purposes such as heating or lighting. Combustion is the burning of fuel with energy released as a byproduct. Fuel is a very important substance for the existence of a modern man. Examples of fuels include petroleum products (petrol, diesel, fuel oil, kerosene, spirits, etc), natural gas, coal, wood, charcoal, producer gas, water gas, etc.
Fuel Sources
Different Sources of Fuels
Identify different sources of fuels
There are many types of substances that are used as fuels. The fuels exist as solids, liquids or gases. The most common substances that are used as fuels in Tanzania include wood, wood charcoal, coal, petroleum products and natural gas. These fuels are obtained from different sources as analysed below:
- Wood: wood is obtained from logs or poles of trees. The wood used as fuel in Tanzania is obtained from natural and artificial forests. Wood fuel is mainly used in rural areas where there are no alternative fuels. Wood is also a major source of fuel used by government institutions such as schools, colleges, hospitals, and military institutions.
- Charcoal: This fuel is made by heating certain substances such as wood and bones in a limited supply of air. Wood charcoal is the main source of fuel in urban areas and in some townships.
- Coal: coal used in Tanzania is mined at Kiwira coal mines. It is used indirectly for generating electricity or directly for powering machines in processing and manufacturing industries and factories. The electricity generated from coal is used in such industries as Tanga cement and several other industries in Dar es Salaam.
- Natural gas: This gaseous fuel is mined at Songosongo in Kilwa (Lindi region), located in southern Tanzania. The gas is used as a fuel at homes and in small industries. It is also used to generate electricity that is used in various manufacturing and processing industries. The electricity generated from this gas is also sold to Tanzania Electricity Supply Company (TANESCO) who distributes the energy to its various clients.
- Petroleum products (kerosene, diesel, petrol, fuel oil, fuel gas, etc.)These petroleum fractions are obtained from crude oil by the process of fractional distillation of crude oil (petroleum). Diesel, petrol and oil are used in vehicles and other machines. Kerosene is used in kerosene lamps and stoves for heating at homes and for other general purposes.
Methods of Obtaining Fuels from Locally Available Materials
Describe methods of obtaining fuels from locally available materials
Methods of making charcoal
When we heat certain organic matter in a limited supply of air, we obtain a black, solid residue called charcoal. The organic matter can be from plant or animal sources for example, wood or animal bones. Heating a substance in limited supply of air is called destructive distillation.
Wood or bone charcoal is made by the process of destructive distillation of wood or bones respectively. Charcoal is largely pure carbon. The entry of air during carbonization (destructive distillation) process is controlled so that the organic material does not burn down to ash as in conventional fire, but instead decompose to form charcoal.
Procedure for making wood charcoal
- Cut wood into small pieces.
- Arrange the wood pieces into a pile of wood on the ground.
- Cover the pieces of wood with soil, leaving one open space for setting fire.
- Set fire to the wood and then cover the open space with soil. Make sure that the wood is burning.
- After the wood is burned, uncover the soil and pull out the black solid substance underneath. This is the charcoal.
Coal formation
Coal is formed from the remains of lush vegetation that once grew in warm shallow coastal swamps. The following are the stages in the process of coal formation:
- The dead vegetation collects in the bottom of the swamp. It may start to decay. But decay soon stops, because the microbes that cause it need oxygen, and the oxygen dissolved in the stagnant, warm water is quickly depleted.
- The vegetation is buried under debris.
- Over hundreds of thousands of years, the environment changes. Seas flood the swamps. Heavy layers of sediment pile up on the dead vegetation, squeezing out gas and water and turning it intopeat.
- As the peat is buried deeper, the increasing heat and pressure compress it progressively to form different types of coal.
- As the process continues, the coal gets harder and more compact. Its carbon content also increases, giving different types of coal. Table bellow shows a summary of the stages in the process:
Stages of formation of different types of coal
| Name of coal | Carbon content | ||
| Peat | 60% | ||
| Pressure and Heat | Lignite | 70% | Hardness |
| Bituminous coal | 80% | ||
| Anthracite | 95% |
As carbon content increases so does energy given out per unit weight. But hard coal tends to have higher sulphur content,hence likely to cause environmental pollution. When burnt, the sulphur in the coal produces sulphur dioxide gas that is released into the atmosphere, causing air pollution.S(s)+O2(g)->S02(g)
Categories of Fuels
Fuels can be classified into three groups according to the physical state of the fuel. A fuel can be in any of the three states of matter namely, solid, liquid or gaseous state.
Fuels According to their States
Classify fuels according to their states
Solid fuels
Solid fuels include wood, charcoal, peat, lignite, coal, coke, etc. The immediate use of all these fuels is for heating and lighting. However, these fuels have a long history of industrial use. Coal was the fuel for the industrial revolution, from firing furnaces to running steam locomotives and trains. Wood was extensively used to run locomotives. Coal is still used for generation of power until now. For example, in Tanzania the coal mined at Kiwira is used for generation of electricity. Also Tanga Cement Company uses coal as a source of power to run machines for production of cement.
Wood is used as a solid fuel for cooking, heating or, occasionally, as a source of power in steam engines. The use of wood as a fuel source for home heating is as old as civilization itself. Wood fuel is still common throughout much of the world. It is the main source of energy in rural areas.
Wood charcoal yields a large amount of heat in proportion to its quantity than is obtained from a corresponding quantity of wood, and has a further advantage of being smokeless. Wood charcoal is often used for cooking and heating, in blacksmithing, etc.
Animal charcoal is used for sugar refining, water purification, purification of factory air and for removing colouring matter from solutions and from brown sugar. Animal charcoal is made by destructive distillation of animal bones.
Coke is a fuel of great industrial use. Coke is obtained by destructive distillation of coal. Most of the coke produced in industry is used as a reducing agent in the production of metals such as pig iron. A substantial amount of coke is also used for making industrial gases such as water gas and producer gas.
Coke is a better fuel than coal because when it is burning, it produces a clean and smokeless flame. When coal is used as a fuel, it produces many toxic gases during burning. Coke has high heat content and leaves very little ash.
Coal is a complex mixture of substances, and its composition varies from one place to another. It depends on coal's age and condition under which it was formed. Anthracite is a very hard black coal and it is the oldest of all types of coal.
When coal is heated in a limited supply of air, it decomposes. This thermal decomposition is called destructive distillation of coal. The products are coke, coal tar, ammoniacal liquor and coal gas.
Liquid fuels
Liquid fuels include petrol (gasoline) diesel, alcohol (spirit), kerosene (paraffin), liquid hydrogen, etc. Liquid fuels have advantage over solid fuels because they produce no solid ashes, and can be regulated by automatic devices. They are relatively more convenient to handle, store and transport than solid fuels.
Most liquid fuels in wide use are derived from fossils. Fossil fuels include coal, natural gas and petroleum. These fuels are formed from remains of sea plants and animals which lived millions of years ago. The remains became buried under layers of sediment. Immense heat and pressure resulted in the formation of coal gas and oil.
Energy produced when petroleum products (diesel, petrol, kerosene, natural gas etc) are burned, originated from the sun. This energy was transferred to animals through their consumption of plants or plant products. When the animals died, got buried, and compressed by heat and pressure, they produced oil which gives off that energy when burnt.
Petroleum fuels are used in cars and in various other machines. Fuels used in cars and lories (petrol and diesel), kerosene (for jet aircraft) and fuel oil (for ships), all came from crude oil. Some oil fuel is also used for electricity generation.
Ethanol burns with a clean, non-smoky flame, giving out quite a lot of heat. On a small scale, ethanol can be used as methylated spirit (ethanol mixed with methanol or other compounds) in spirit lamps and stoves. However, ethanol is such a useful fuel that some countries have developed it as a fuel for cars. In countries where ethanol can be produced cheaply, cars have been adapted to use a mixture of petrol and ethanol as fuel.
Brazil has a climate suitable for growing sugarcane. Ethanol produced by fermentation of sugarcane has been used as an alternative fuel to gasoline (petrol), or mixed with gasoline to produce "gasohol". Currently, about half of Brazil’s cars run on ethanol or "gasohol". "Gasohol" now accounts for 10% of the gasoline sales in the U.S.A.
The idea about the use of biofuel for fuelling automobiles and other machines has been borrowed by other countries including Tanzania. However, the programme has raised a bitter concern among different activists. Their doubt is that emphasis on growing crops for biofuel production may take up land that could otherwise be used for growing food crops. This, therefore, would mean that there would not be enough land to grow enough food to feed the ever-increasing human population. Hence, hunger will prevail. Notwithstanding all these shouting, biofuel crop production is there to stay!
Gaseous fuels
The use of gaseous fuels for domestic heating is common in urban areas. Compressed gas that is delivered to our homes in steel cylinders is liquefied propane, butane, or mixture of the two. When the valve is opened, the liquid gas vapourizes quickly into gas and passes through a pipe to the stove. Gaseous fuels are the most convenient fuels to handle, transport and store.
The following is a list of types of gaseous fuels:
- Fuel naturally found in nature: -natural gas -methane from coal mine
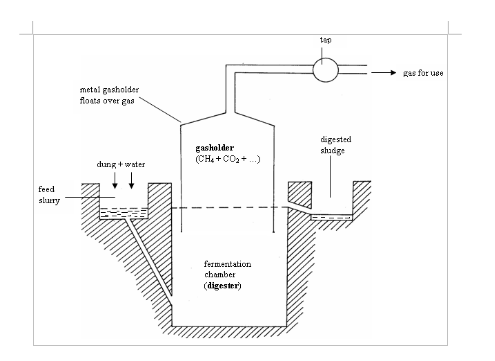
- Fuel gas from solid fuels or materials: -gas derived from coal (water gas and producer gas) -gas derived from wastes and biomass (biogas)
- Fuel gas made from petroleum.
Gaseous fuels used in industry
Producer gas and water gas are important industrial fuels.
Producer gas
Producer gas is produced by burning a solid carbonaceous fuel, such as coke, in a limited supply of air in a producer furnace. The reaction is exothermic and this makes coke to get hotter. Carbonaceous fuels are fuels that contain a high proportion of carbon. The producer gas is a mixture of carbon monoxide and nitrogen.
When air, mixed with a little steam, is passed through the inlet in the lower part of the furnace, the coke (carbon) combines with oxygen (from air) to form carbon dioxide:
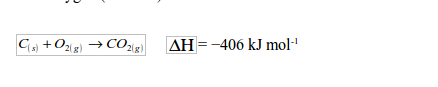
As the carbon dioxide formed rises up through the red-hot coke, it is reduced to carbon monoxide:

Since more heat (406 kJ) is produced in the lower part than is absorbed in the upper part of the furnace (163 kJ), some excess heat is obtained in the long run. This heat keeps the coke hot. The nitrogen gas in the air is not affected at all during the process. Hence, the overall reaction equation may be represented as follows:
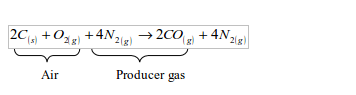
As a fuel, producer gas burns to give out carbon dioxide.

Because a good deal of producer gas contains nitrogen, a gas that does not support combustion, it has a lower calorific value compared to water gas. See table 4.2 for comparison.
Water gas
Water gas is produced by passing steam over white-hot coke at 1000°C. The gas is a mixture of hydrogen and carbon monoxide. The reaction is endothermic, causing the coke to cool.

Water gas burns as a fuel to give carbon dioxide and steam.
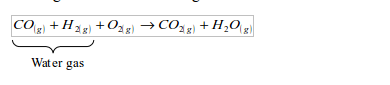
However, carbon monoxide is a very poisonous gas. The gas made from petroleum or coal contains some carbon monoxide, which makes it poisonous. Natural gas is safer and efficient, as it contains no carbon monoxide.
Characteristics of a good fuel
A good fuel burns easily to produce a large amount of energy. Fuels differ greatly in quality. There are certain characteristics, which make a good fuel. After all, there is no fuel among the different fuels known that posses all the virtues that a good fuel should have. Generally, a good fuel has the following
characteristics:
- It should be environmentally friendly (not harm the environment) in the course of its production and use, that is, it should not produce harmful or toxic products such as much smoke, carbon dioxide, carbon monoxide, sulphur dioxides, etc, which pollutes the air.
- It must be affordable to most people i.e. it must be cheap.
- It should not emit or produce dangerous by-products such as poisonous fumes, vapour or gases.
- It should have high calorific value i.e. it must burn easily and produce a tremendous quantity of heat energy per unit mass of the fuel.
- It should be easy and safe to transport, store, handle and use.
- It should be readily available in large quantities and easily accessible.
- It should have high pyrometric burning effect (highest temperature that can be reached by a burning fuel). Normally gaseous fuels have the highest pyrometric effect as compared to liquid and solid fuels.
- It should have a moderate velocity of combustion (the rate at which it burns) to ensure a steady and continuous supply of heat.
- A good fuel should have an average ignition point (temperature to which the fuel must be heated before it starts burning). A low ignition point is not good because it makes the fuel catch fire easily, which is hazardous, while high ignition point makes it difficult to start a fire with the fuel.
- A good fuel should have a low content of non-combustible material, which is left as ash or soot when the fuel burns. A high content of no-combustible material tends to lower the heat value of the fuel.
Calorific values of fuels
The heating value or calorific value of a substance, usually a fuel or food, is the amount of heat released during the combustion of a specific amount of it. The calorific value is a characteristic of each substance. It is measured in units of energy per unit of substance, usually mass, such as Kcal/Kg, J/g, KJ/Kg, KJ/Mol, MJ/m3, etc. Heating value is commonly determined by use of an instrument called bomb calorimeter.
By custom, the basic calorific value for solid and liquid fuels is the gross calorific value at constant volume, and for gaseous fuels, it is the gross calorific value at constant pressure.
Calorific values of solid, liquid and gaseous fuels
| Solid and liquid fuels | Calorific value (MJ/kg) |
| Alcohols | |
| Ethanol | 30 |
| Methanol | 23 |
| Coal and coal products | |
| Anthracite (4% water) | 36 |
| Coal tar fuels | 36 - 41 |
| General purpose coal (5-10% water) | 32 - 42 |
| High volatile coking coals (4% water) | 35 |
| Low temperature coke (15% water) | 26 |
| Medium-volatile coking coal (1% water) | 37 |
| Steam coal (1% water) | 36 |
| Peat | |
| Peat (20% water) | 16 |
| Petroleum and petroleum products | |
| Diesel fuel | 46 |
| Gas oil | 46 |
| Heavy fuel oil | 43 |
| Kerosene | 47 |
| Light distillate | 48 |
| Light fuel oil | 44 |
| Medium fuel oil | 43 |
| Petrol | 44.80 - 46.9 |
| Wood | |
| Wood (15% water) | 16 |
| Gaseous fuels at 15ºC, 101.325 kPa, dry | Calorific value (MJ/m3) |
| Coal gas coke oven (debenzolized) | 20 |
| Coal gas low temperature | 34 |
| Commercial butane | 118 |
| Commercial propane | 94 |
| North sea gas, natural | 39 |
| Producer gas coal | 6 |
| Producer gas coke | 5 |
| Water gas carburetted | 19 |
| Water gas blue | 11 |
Measuring the heat given out by fuels
We burn fuels to provide us with heat energy. The more heat a fuel gives out the better. The amount of heat given out when one mole of fuel burns is called heat of combustion. This is often written as
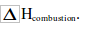
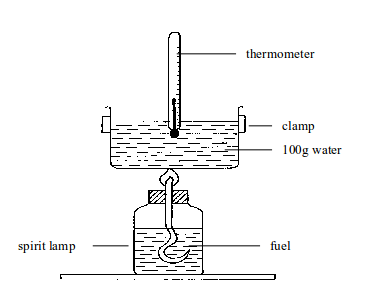
This value can be measured in the laboratory indirectly by burning the fuel to heat water. Simple apparatus is shown in figure bellow. The basic idea is: Heat gained by the
Heat gained by the water = heat given out by the fuel.
Method
These are the steps:
- Pour a measured volume of water into the tin. Since you know its volume you also know its mass (1 cm3 of water has a mass of 1g).
- Weigh the fuel and its container.
- Measure the temperature of the water.
- Light the fuel and let it burn for a few minutes.
- Measure the water temperature again, to find the increase.
- Reweigh the fuel and container to find how much fuel was burned.

Measuring the energy value of a fuel
Calculations
It takes 4.2J of energy to raise the temperature of 1g of water by 1ºC. This constant value is called specific heat capacity of water, usually represented as 4.2Jg-1C-1(4.2 joules per gram per centigrade). So, you can calculate the energy given out when the fuel burns by using this equation:
Energy given out = 4.2g-1C-1 mass of water (g) its rise in temperature (ºC).
Then since you know what mass of fuel you burned you can work out the energy that would be given out by burning one mole of it.
Example 1
The experiment gave these results for ethanol and butane. Make sure you understand the calculations:
Experimental results for heat determination
| Ethanol (burned in a spirit lamp) | Butane (burned in a butane cigarette lighter) |
| Results | Results |
| Mass of ethanol used: 0.9g | Mass of butane: 0.32g |
| Mass of water used: 200g | Mass of water used: 200g |
| Temperature rise: 20ºC | Temperature rise: 12ºC |
| Calculations | Calculations |
| Heat given out = or 16.8KJ | Heat given out = = 10080J or10.08KJ |
| The formula mass of ethanol is 46. 0.9g gives out 16.8KJ of energy. So, 46g gives out of energy | The formula mass of butane is 58. 0.32 gives out 10.08KJ of energy. So, 58g gives out KJ of energy |
| So, H combustion for ethanol is -859KJ/mol | So, H combustion for butane is -1827 KJ/mol |
Example 2
Determination of energy (calorific) value of ethanol
The energy/heating/calorific value of a fuel refers to the amount of heat given out when a specific amount of fuel is burned.
Experiment
Aim: To find out the energy value of ethanol.
Materials: water, beaker, thermometer, weighing balance, spirit lamp and ethanol.
Procedure:
- Pour a known volume of water into a beaker.
- Measure the temperature of the water.
- Fill the spirit lamp with enough ethanol.
- Weight the mass of both the ethanol and the lamp.
- Light the lamp and let it continue burning for a few minutes before putting it off.
- Measure the water temperature again, to find the increase.
- Reweigh the ethanol and its container to find how much ethanol was burned.
Record the following:
- Mass of spirit lamp + ethanol (initially)
- Mass of spirit lamp + ethanol (finally)
- Mass of ethanol burned
- Final temperature of water
- Initial temperature of water
- Rise in temperature of water
- Mass of water
The amount of heat (q) released by ethanol is given by:

Specimen calculation:
Mass of lamp and ethanol initially = 50g
Mass of lamp and ethanol finally = 49.5g
Mass of ethanol burned = 50.0 — 49.5 = 0.5g
Mass of water = 100g
Final temperature of water = 42ºC
Initial temperature of water = 20ºC
Rise in temperature = 42ºC – 20ºC = 22ºC
Specific heat capacity of water = 4.2 Jg-1C-1
Heat given out = Mass of water Xspecific heat capacity Xtemperature rise
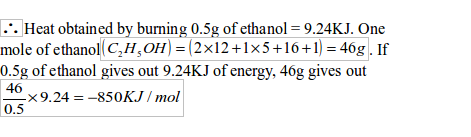
Repeat similar procedures with kerosene, charcoal, coal, firewood etc. and compare your results. Which fuel has more energy per gram? That is the most efficient fuel.
How reliable is the experiment?
The following table compares the experimental results with values from data book.
| Fuel | Heat of combustion in KJ/mol | |
| From the experiment | From a data book | |
| Ethanol | -859 | -1367 |
| Butane | -1827 | -2877 |
Note the big difference! The experimental results are almost 40% lower for both fuels. Why do you think there is such a big difference? There are two reasons for this:
- Heat loss: Not all the heat from the burning fuel is transferred to the water. Some is lost to the air, and some to the container that holds the fuel.
- Incomplete combustion: In case of a complete combustion, all the carbon in a fuel is converted to carbon dioxide. But here combustion is incomplete. Some carbon is deposited as soot on the bottom of the lamp and some converted to carbon monoxide. For example, when butane burns, a mixture of all these reactions may take place:
The less oxygen there is, the more carbon monoxide and carbon will form.
Conservation of Energy
What is energy?
Energy is defined as the ability to do work or bring about change. Energy makes changes; it does things for us. It moves cars along the road, and boats over the water. It bakes cakes in the oven and keeps ice frozen in the freezer. It plays our favourite songs on the radio and lights our homes. Energy makes our bodies grow and allow our minds to think. People have learned how to change energy from one form to another so that we can do work more easily and live more comfortably. The source of all energy on earth is the sun.
Forms of energy
Energy exists in many different forms such as heat, light, sound, electrical, etc. The amount of energy can be measured in joules, kilojoules, megajoules, calories, etc. There are many forms of energy, but they can all be put in two categories: Kinetic and Potential.
Forms of energy
| KINETIC ENERGY | POTENTIAL ENERGY |
| Kinetic energy is energy in motion of waves, electrons, atoms, molecules, substances, and objects. | Potential energy is stored energy and the energy of position - gravitational energy. |
| Electrical energyis the movement of electrical charges. Everything is made of tiny particles called atoms. Atoms are made of even smaller particles called electrons, protons and neutrons. Applying a force can make some of the electrons move. Electrical charges moving through a wire is called electricity. Lightning is another example of electrical energy. | Chemical energy is energy stored in the bonds of atoms and molecules. This energy holds these particles together. Biomass, petroleum, natural gas, and propane are examples of stored chemical energy. |
| Radiant energy is electromagnetic energy that travels in transverse waves. Radiant energy includes visible light, x-rays, gamma rays and radio waves. Light is one type of radiant energy. Solar energy is an example of radiant energy. | Stored mechanical energy is energy stored in objects by the application of a force. Compressed springs and stretched rubber bands are examples of mechanical energy. |
| Thermal energy,or heat energy, is the internal energy in substances caused by the vibration and movement of the atoms and molecules within substances. Geothermal energy is an example of thermal energy. | Nuclear energy is energy stored in the nucleus of an atom - the energy that holds the nucleus together. The energy can be released when the nuclei are combined or when a nucleus splits apart (disintegrates). Nuclear power plants split the nuclei of uranium atoms in a process called fission. The sun combines the nuclei of hydrogen atoms in a process called fusion. Scientists are working creating fusion energy on earth, so that someday there might be fusion power plants. |
| Motion energy is the energy which enables movement of objects and substances from one place to another. Objects and substances move when a force is applied according to Newton's laws of motion. Wind is an example of motion energy. | Gravitational energy is the energy of position or place. A rock resting at the top of a hill contains gravitational potential energy. Hydropower, such as water in reservoir behind a dam, is an example of gravitational potential energy. |
| Sound energy is the movement of energy through substances in longitudinal (compression/rarefaction) waves. Sound is produced when a force causes an object or substance to vibrate - the energy is transferred through the substance in a wave |
Kinetic energy is energy in motion. Its existence can be shown by winds, ocean currents, running water, moving machines or a falling body.
Potential energy is energy at rest. It is found stored in different forms, e.g. in coal, petroleum and natural gas, batteries and muscles. Such energy does not work so long as it is stored. It is capable of doing work when it is converted to other forms of energy such as heat, light or radiation.
The Law of Conservation of Energy
Explain the law of conservation of energy
Energy conversion (Energy changes)
Can energy be created or destroyed? When wood or charcoal is burned, it appears as if energy is destroyed and wasted. In fact, the energy in these kinds of fuels is not destroyed when the fuels are burned. It is simply converted to other forms of energy such as heat and light.
When you are seated on a desk in class, you are possessing potential energy. When you stand up and walk away from the classroom, you are transforming the potential (chemical) energy in your muscles to kinetic energy.
The Law of Conservation of Energy states that energy can neither be created nor destroyed but it can only be changed from one form to another. When we use energy, it does not disappear. We simply convert it from one form to another.
Potential (chemical) energy in a dry cell is converted to electrical energy which is finally converted to sound energy in radio speakers. In a tape record player, the same chemical energy is ultimately converted to kinetic energy to drive the cassettes. When the potential energy is all used up, the batteries are dead. In the case of rechargeable batteries, their potential energy is restored through recharging.
The chemical energy in your mobile phone battery can be converted into sound, light, text, etc. The main energy changes that occur in a variety of simple situations are:
- Battery chemical to electrical, sound or light;
- Car engine chemical to mechanical and then kinetic;
- Light bulb electrical to light and heat;
- Parachutist potential to kinetic;
- Solar heat to electrical and kinetic;
- Wind mill kinetic to electrical;
- Running water kinetic to electrical;
- Muscles chemical to kinetic, etc.
What other situations of energy changes do you know? Mention them
All energy changes that occur during chemical and physical changes must conform to the Law of Conservation of Energy, that is, energy can only be changed from one form into its equivalent of another form with no total loss or gain.
The most common form of energy in chemistry is the heat change. A chemical reaction must involve some change in energy. As the reaction occurs, chemical bonds of reactant molecules are broken while those of the product molecules are formed. Energy is given out when a chemical bond forms and it is consumed when a bond is broken.
Take an example of combustion (respiration) of glucose in living cells:
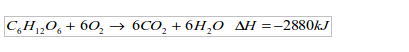
During respiration process, the bonds of glucose and oxygen are broken down while those of carbon dioxide and water are formed. Heat is absorbed when chemical bonds are broken and it is released when the bonds are formed. The total amount of heat absorbed by the reactants is equal that released by the products. Heat absorbed is given a positive sign (+ve) while heat given out is assigned a negative sign (-ve). So the total energy change is equal to zero. This means that no energy has been created or destroyed.
Experiments on the Conservation of Energy from One Form to Another
Carry out experiments on the conservation of energy from one form to another
Activity 1
Carry out experiments on the conservation of energy from one form to another.
Renewable Energy Biogas
Renewable energy sources include biomass, geothermal energy, hydroelectric power, solar energy, wind energy, and chemical energy from wood and charcoal. These are called renewable energy sources because they are replenished within a short time. Day after day, the sun shines, wind blows, river flows and trees are planted. We use renewable energy sources mainly to generate electricity.
In Tanzania most of the energy comes from non-renewable sources. Coal, petroleum, natural gas, propane and uranium are examples of non-renewable energy sources. These fuels are used to generate electricity, heat our homes, move our cars and manufacture many kinds of products. These resources are called non-renewable because they cannot be replenished within a short time. They run out eventually. Once, for example, coal or petroleum is depleted, it may take millions of years to be replaced. So, these are non-renewable energy sources.
BIOGAS
Biogas is a gaseous fuel produced by the decomposition of organic matter (biomass). Under anaerobic conditions, bacteria feed on waste organic products, such as animal manure and straw, and make them decay. The product formed from this decay is called biogas, which consists mainly of methane, though other gases such as carbon dioxide, ammonia, etc, may also be produced in very small quantities. The biogas produced can be used as a fuel for cooking, heating, etc.
Raw materials for biogas production may be obtained from a variety of sources, which include livestock and poultry wastes, crop residues, food processing and paper wastes, and materials such as aquatic weeds, water hyacinth, filamentous algae, and seaweeds.
The Working Mechanism of Biogas Plant
Explain the working mechanism of biogas plant
The organic waste products are fed in a biogas plant. Prior to feeding the material into the plant, the raw material (domestic poultry wastes and manure) to water ratio should be adjusted to 1:1 i.e. 100 kg of excreta to 100 kg of water. Then adequate population of both the acid-forming and methanogenic bacteria are added.
The bacteria anaerobically feed on the liquid slurry in the digester. The major product of this microbial decomposition is biogas, which largely contain methane gas. The gas so produced is collected in the gas holder and then taped off. The gas is used as a fuel for cooking, heating and other general purposes.
The biological and chemical conditions necessary for biogas production
Domestic sewage and animal and poultry wastes are examples of the nitrogen-rich materials that provide nutrients for the growth and multiplication of the anaerobic organisms. On the other hand, nitrogen-poor materials like green grass, maize stovers, etc are rich in carbohydrates that are essential for gas production. However, excess availability of nitrogen leads to the formation of ammonia gas, the concentration of which inhibits further microbial growth. This can be corrected by dilution or adding just enough of the nitrogen-rich materials at the beginning.
In practice it is important to maintain, by weight, a C:N close to 30:1 for achieving an optimum rate of digestion. The C:N can be manipulated by combining materials low in carbon with those that are high in nitrogen, and vice versa.
A pH range for substantial anaerobic digestion is 6.0 – 8.0. Efficient digestion occurs at a pH near to neutral (pH 7.0). Low pH may be corrected by dilution or by addition of lime.
To ensure maximum digestion, stirring of the fermentation material is necessary. Agitation (stirring) can be done either mechanically with a plunger or by means of rotational spraying of fresh organic wastes. Agitation ensures exposure of new surfaces to bacterial action. It also promotes uniform dispersion of the organic materials throughout the fermentation liquor, thereby accelerating digestion.
A Model of Biogas Plant
Construct a model of biogas plant
The biogas plant consists of two components: the digester (or fermentation tank) and a gas holder. The digester is a cube-shaped or cylindrical waterproof container with an inlet into which the fermentable mixture is introduced in the form of liquid slurry. The gas holder is normally an airproof steel container that floats on the fermentation mix. By floating like a ball on the fermentation mix, the gas holder cuts off air to the digester (anaerobiosis) and collects the gas generated. As a safety measure, it is common to bury the digester in the ground or to use a green house covering.

Structure of the biogas plant
The Use of Biogas in Environmental Conservation
Explain the use of biogas in environmental conservation
Environmental conservation is a major concern in life. We need to live in a clean and health environment so as to enjoy our lives better. The use of biogas as an alternative source of energy is essential in environmental conservation due to a number of reasons. These are some of the reasons:
- Biogas does not produce much smoke or ash, which could otherwise pollute the atmosphere or land. When the gas is burned it produces very little smoke and no ash as compared to other sources of fuel such as wood.
- The use of biogas for cooking and heating prevents the cutting down of trees to harvest firewood, or burn charcoal for fuel, a practice that could result to soil erosion, drought, etc. Hence, using the biogas as fuel helps to conserve the environment as no more cutting of trees may be done.
- Using cow dung, poultry manure and other excreta for biogas production helps keep the environment clean because these materials are put into alternative use instead of just being dumped on land, a fact that could lead to pollution of the environment.
- Some biomass employed in biogas production is toxic and harmful. By letting these materials be digested by bacteria, they may be turned into non-toxic materials that are harmless to humans, plants, animals and soil.
- The excreta used for production of biogas produce foul smell if not properly disposed of. Using this excrete to generate biogas means no more bad smell in air.
- Health hazards are associated with the use of sludge from untreated human excreta as fertilizer. In general, a digestion time of 14 days at 35ºC is effective in killing the enteric bacterial pathogens and the enteric group of viruses. In this context, therefore, biogas production would provide a public health benefit beyond that of any other treatment in managing the rural health and environment of developing countries.
The Atom
We learned early that matter is made up of small, indivisible particles. Everything around us is made of extremely small particles. These particles are either molecules or atoms. An atom is the smallest indivisible particle of an element that takes part in a chemical change. Atoms are the building blocks of matter. All solids, liquids and gases are made of atoms fitted in different ways.
The present day chemistry is built on the foundations of the Atomic Theory. The idea that elements are made up of atoms is called the Atomic Theory. An English chemist, John Dalton was the first to put forward the Atomic Theory, which for most of the 19thcentury stated that atoms were hard, extremely small, indivisible and spherical particles like minute lead shots
Dalton Contribution to Atomic Structure
Explain Dalton contribution to atomic structure
The Greek philosopher Democritus (460-370 BC) believed that matter was indestructible and that it is made up of tiny particles called atoms. Our modern understanding is based on the Atomic Theory which was put forward by John Dalton in 1808. His theory re-introduced the ideas of Democritus and other Greek philosophers who suggested that all matter was infinitely divided into very small particles called atoms. These ideas were not widely accepted at that time. They were only revived when Dalton developed them further and experimental science was able to back them up with practical observations.
The Atomic Theory goes back to ancient Greeks, yet we always talk today about Dalton's Atomic Theory. There is a good reason for this. The reason is that, while Greeks put forward the idea that atoms exist they did nothing more. They left the idea vague and untested. Dalton changed this vague imaging into a set of concrete suggestions about atoms which could be tested by experiment. This change from vagueness to precision and experimental test justifies his claim to the theory.
Dalton’s Atomic Theory contains the following main ideas:
- Matter is made up of small, indivisible particles called atoms.
- Atoms of the same element are all exactly alike in every way and have definite weights.
- Atoms are indestructible and they cannot be created.
- Atoms of different elements have different weights and posses different properties.
- Atoms of different elements combine in small whole numbers to form ‘compound atoms’.
The Modern Concept of Dalton’s Atomic Structure
Explain the modern concept of Dalton’s atomic structure
From the theory, it is observed that each atom has its own mass and that chemical combination takes place between atoms and not fractions of atoms.
Discoveries made in the 20th century, however, showed that certain parts of the theory must be modified. However, Dalton‟s Theory was one of the great leaps of understanding of chemistry. It meant that we could explain many natural processes.
Dalton's Atomic Theory was the first step towards the formation of Modern Atomic Theory. The Dalton's Theory has been subjected to numerous experimentations that have led to some modifications to the theory. However, some ideas in his theory still hold strongly to date. Some modifications to the theory include the following:
- The atom is no longer regarded as indivisible, or the smallest particle. Particles smaller than the atom; electrons, protons and neutrons are now known. However, the atom is still the smallest particle which can take part in a chemical reaction.
- Atoms of the same element may not be all alike. Some elements have atoms with different atomic masses e.g. carbon 12 and carbon 14. These different atoms of the same element are called isotopes.
- In some few cases, atoms of different elements may have the same atomic mass. Both argon and calcium have atomic mass 40. Such atoms are called isobars.
- "The compound atoms" of Dalton are known as molecules. A molecule is the simplest particle of matter which is capable of independent existence. Evidence is available where atoms of different elements combine in large integers. An example is in organic and silicon compounds.
- Atoms are no longer regarded as indestructible. Radioactive atoms may get destroyed by spontaneous decay or by atomic fission.The atom is therefore the smallest particle of an element which is responsible for the chemical properties of that element, and which takes part in a chemical reaction.
Sub-atomic Particles
Sub-atomic Particles in an Atom
Identify sub-atomic particles in an atom
Dalton thought that atoms were solid, indivisible particles. But, as a result of work done mainly by Lord Rutherford, the idea has been greatly changed in recent years. According to Rutherford, the atom consists of 3 kinds of particles - protons, neutrons and electrons. These are called sub-atomic particles.
The centre of the atom is called nucleus. The nucleus contains a cluster of two sorts of particles, protons and neutrons. Thenucleus is very small, occupying only about 1% of the volume of an atom. The rest of the atom is mostly empty space, withelectrons spread out in it.

Electrons move around the nucleus in special paths calledelectron shells (orbits/or orbitals or energy levels). Protons andelectrons have electric charges. Neutrons have no charges.All the particles in an atom are very light. Their masses aremeasured in atomic mass units rather than grams. The proton isa positively charged particle. Its mass is about equal to that ofhydrogen atom. The neutron is has no charge, it is neutral. Itsmass is about equal to that of hydrogen atom. The electron isnegatively charged. Its charge is equal but opposite to the chargeon the proton. It has a very small mass, about 1⁄1840 of the massof the proton.
The Properties of each Particle in an Atom
Explain the properties of each particle in an atom
The properties of these particles are summarizedin the table below:
Table 5.1: Properties of sub-atomic particles

A single atom is electrically neutral (it has no electrical charge).This means that in any atom there must be equal numbers ofprotons and electrons. In this way, the total positive charge onthe protons is balanced by the total negative charge on theelectrons orbiting the nucleus. So, the charges must cancel.
Electronic Arrangements
Electronic arrangement refers to the manner in which electronsare arranged in an atom. An atom contains a central nucleuscontaining protons and neutrons, and a cluster of electronsrevolving in orbits around the nucleus. These electrons aregrouped in shells.
A Maximum Number of Electrons in the Shells
Determine a maximum number of electrons in the shells
Bohr (1913) put forward a theory of electron positioning whichis still generally accepted and used until now for chemicalpurposes. Bohr's Theory on the arrangement of electrons in anatom can be summarized as follows:
- Electrons are in orbit around the nucleus of the atom.
- The electron orbits are grouped together in shells; a shellis a group of orbits occupied by electrons withapproximately equal energy.
- The electrons in shells distant from the nucleus havehigher energy than those in shells close to the nucleus.
- Electrons fill the shells starting with the first shell, whichis closest to the nucleus. Shells are numbered 1, 2, 3, 4,98etc. outwards from the nucleus. The shells may berepresented by the letters K, L, M and N respectivelystarting from the nucleus.
- The maximum possible number of electrons in a shellnumbered n is 2 2n .
- The first shell can only contain up to 2 electrons. Thesecond shell can contain a maximum of 8 electrons. Thethird shell can contain up to 18 electrons.
- In the outermost shell of any atom, the maximumnumber of electrons possible is 8.
- The outer electrons of some atoms can be removed fairlyeasily to form ions.
- Chemical bonding between atoms to form moleculesinvolves the electrons in the outer shell only.
Electronic arrangement of some typical atoms is shown in figure5.2.

The arrangement of electrons around the nucleus is also knownas electronic configuration. This arrangement depends on themaximum number of electrons that can occupy a shell. An atomwith 13 electrons will have the following electronicconfiguration: 2:8:3. This means that there are 2 electrons in thefirst shell, 8 electrons in the second shell and 3 electrons in the third shell.
The number and arrangement of electrons in the atoms of the first 20 elements are shown in table 5.2.
Table 5.2: The electron arrangements of the first 20 elements

After the first 20 elements, the organization of the electrons becomes increasingly complicated. The third shell (n = 3) can be occupied by a maximum number of 18 electrons
At this stage, you will not be asked to work out electronarrangements beyond element 20 (calcium), but you should beable to understand the electronic structures involving moreelectrons (for example bromine with the arrangement 2:8:18:7).
Energy Shell Diagrams
Draw energy shell diagrams
Energy Shell Diagram

Atomic number, Mass number and Isotope
Relationship between Atomic Number and Number of Protons
Relate atomic number with number of protons
All atoms of one element have the same number of protons. Thisis called the atomic number (or proton number) of that element.It is given by the symbol Z.
No two elements can have the same atomic number. Sodiumatoms have 11 protons. This is what makes them different fromall other atoms. Only sodium atoms have 11 protons, and anyatom with 11 protons must be sodium atom.
In the same way, an atom with 6 protons must be carbon atom.Also any atom with 7 protons must be nitrogen atom. So, youidentify an atom by the number of protons in it. There are 109elements altogether. Of these, hydrogen has the smallest atoms,with only 1 proton each. Helium atoms have 2 protons each.Lithium atoms have 3 protons each, and so on up to meitneriumatoms, which have 109 protons each. Table 5.3 shows the first20 elements arranged according to the number of protons theyhave.
Every atom has an equal number of protons and electrons, so theatomic number also tells us the number of electrons in that atom.In any given atom of an element, the number of neutrons has no effect on the identity and properties of that particular element. It is the number of protons and electrons that determine the identity and properties of any given element. The number of neutrons only affects the mass, since each one of them has the same mass as that of a proton.
Mass Number of an Atom from Numbers of Protons and Neutrons
Calculate mass number of an atom from numbers of protons and neutrons
Protons alone do not make up all the mass of an atom. The neutrons in the nucleus also contribute to the total mass. The mass of the electrons can be regarded as so small that it can be ignored. As a proton and a neutron have the same mass, the mass of a particular atom depends on the total number of protons and neutrons present. This is called mass number (or nucleon number). The mass number of an atom is found by adding together the number of protons and neutrons. It is given by the symbol A. Table 5.3 shows the mass number of the first 20 elements, arranged in order of increasing atomic mass (mass number).
If the mass number and atomic number for any given atom are known, then its sub-atomic composition can be worked out.
The mass number = number of protons + neutrons in an atom. Sodium atom has 11 protons and 12 neutrons, so the mass number of sodium is 23. Since the atomic number is the number of protons only, then:
Mass number – atomic number = number of neutrons. So, for sodium atom, the number of neutrons = (23-11) =12. You can also take into account the fact that, because the number of protons is always equal to the number of electrons, then the number of electrons in sodium atom is simply 11. The same rule can be applied to work out the sub-atomic composition of any element.
These two relationships are useful:
- Number of electrons = number of protons = atomic number
- Number of neutrons = mass number (A) – atomic number (Z).

The Concept of Isotope
Explain the concept of isotope
Atoms of the same element may have different numbers of neutrons. In a normal situation, atoms of the same element will have the same number of neutrons. However, many cases occur in which two atoms of the same element contain the same number of protons but different numbers of neutrons. Having equal number of protons, these atoms must also have equal numbers of electrons. However, the differing numbers of neutrons cause the atoms to have different mass numbers. An element showing such properties is said to show isotopy and the varieties of the atom are called isotopes of the element.
Therefore, isotopy can be defined as the tendency of atoms of one element to posses the same atomic number but different mass numbers (atomic masses). Isotopes can be defined as atoms of the same element with the same number of protons but different numbers of neutrons, or as „atoms of the same element with the same atomic number but different atomic masses‟.
The isotopes of an element have the same chemical properties because they contain the same number of electrons. It is the number of electrons in an atom that decides the way in which it forms bonds and reacts with other atoms. However, some physical properties of the isotopes are different. The masses of the atoms differ, and therefore other properties, such as density and rate of diffusion, also vary.
Many isotopes (like tritium) are unstable. The extra neutrons in their nuclei cause them to be unstable so that nuclei break spontaneously (that is, without any extra energy being supplied), emitting certain types of radiation. They are known as radioisotopes.
Notation for isotopes
In order to distinguish between different isotopes of the same element in writing symbols and formulae, a simple system is adopted. The isotope of an element, say X will have the symbol X,AZ , where A is the mass number of the isotope and Z is the atomic number of any atom of X. Thus, for all isotopes of one element, Z is constant, and A varies because there are different numbers of neutrons in the different isotopes of the element. For example, the three isotopes of carbon are expressed as 12C6, 13C6,and 14C6. Chlorine has two isotopes: 35Cl17 and 37Cl17 . Since A represents the total number of neutrons and protons in the nucleus of an atom (mass number/atomic mass), and because Z is the number of protons (atomic number), then the number ofneutrons in the nucleus of a given isotope is given by:Number of neutrons in the nucleus = A – Z
Relative atomic masses
As we have seen, most elements exist naturally as isotopes.Therefore, the value we use for the atomic mass of an element isan average mass. This takes into account the proportions(abundance) of all the naturally occurring isotopes. If aparticular isotope is present in high proportion, it will make alarge contribution to the average.
Example
A sample of chlorine gas contains 75% of the isotope 35Cl17 and 25% of the other isotope 37Cl17 . What is the relative atomic mass of chlorine?
Solution
To work out this problem, simply multiply the mass number ofeach isotope with the abundance and sum up the products thus:
This average value for the masses of atoms of an element isknown as the relative atomic mass (Ar).Therefore, the relative atomic mass of chlorine is 35.5 (i.e., Ar =35.5).
TOPIC 6: PERIODIC CLASSIFICATION
Constructing the modern periodic table has been a major scientific achievement. The first steps towards working out this table were taken long before anyone had any idea about the structure of atoms. The number of elements discovered increased steadily during the 19th century. Chemists began to find out patterns in their properties.
The Law of Triads
In 1817, the German scientist Johann Dobereiner noticed that calcium, strontium and barium had similar properties, and that the atomic weight of strontium was halfway between the other two. He found the same pattern with chlorine, bromine and iodine and also with lithium, sodium and potassium. So, he put forward the law of Triads: “If elements are arranged in groups of three in order of increasing atomic weights, having similar properties, then the atomic weight of the middle element is the arithmetic mean of the atomic weights of the other two elements”, E.g.
The following are examples of Dobereiner's triads:(Lithium, Sodium and Potassium)(Calcium, Strontium and Barium)(Chlorine, Bromine and Iodine) and(Iron, Cobalt and Nickel)
The Law of Octaves
In 1863 John Newlands, an English chemist noted that there were many pairs of similar elements. In each pair, the atomic weights differed by a multiple of 8. So, he produced a table with the elements in order of increasing atomic weights, and put forward the Law of Octaves: “If elements are arranged in order of their increasing atomic weights, the properties of the 8th element, starting from a given one, are a kind of repetition of the first element”.
This finding was comparable to the 8th note of music, hence the use of the word "octave".
This was the first table to show a periodic or repeating pattern of properties. But it was not widely accepted because there were too many inconsistencies. For example, he put copper and sodium in the same group, even though have very different properties. Also iron was placed in the same group as oxygen and sulphur.
The Periodic Law
Dmitri Mendeleev was born in Siberia, Russia, in 1834. By the time he was 32, he was a professor of Chemistry. In 1869 Mendeleev advanced the work done by Newlands and contributed very useful new ideas. He began by listing all the known elements in order of increasing atomic mass. He spotted that elements with similar properties appear at regular intervals or periods down the list. His findings were the basis for the Periodic Law: “The properties of elements are a periodic function of their atomic masses”.
Mendeleev placed similar elements into groups. He realized that not all elements had been discovered. So he left gaps for new ones in the correct places in his table. He also swapped the order of some elements to make them fit better. He predicted the properties of the missing elements from the properties of the elements above and below them in the table. He also listed separately some elements which did not appear to fit into any group i.e. iron, cobalt, nickel, etc.
Table 6.1: Mendeleev’s short form of the Periodic Table

The table had 9 vertical columns which he called Groups. The groups were numbered from 0 to 8. The elements in group 0 were not known by then, but were discovered later on. Groups 1 to 7 were subdivided into A and B subgroups. Group 0 included the transition elements. Noble gases were later placed in group 0.
There were 7 horizontal rows which he called periods. All vacant positions in the table stood for new elements yet to be discovered.
Usefulness of Mendeleev's classification
- The table summarized a large amount of information about the elements based on their chemical properties.
- The table was very useful in predicting the existence and properties of undiscovered elements, for which gaps had been left in the table.
- The table was also used in checking relative atomic masses of elements.
Limitations of Mendeleev's classification
- In three cases, pairs of elements had to be included in one group based on inverse order of their atomic weights so as to fit into groups of elements having similar properties. These pairs were argon (39.9) and potassium (39.1), cobalt (58.9) and nickel (58.9); plus tellurium (127.5) and iodine (126.9). This difficulty was resolved when the basis of classification was based on the atomic number instead of the atomic mass.
- The elements that were placed in group VIII formed an incompatible mixture.
- The placing of two different families in one group e.g. K and Cu; Ca and Zn, etc.
The periodic table is the chemists map. It helps you understand the patterns in chemistry. Today we take it for granted. But it took hundreds of years, and work of hundreds of chemists, to develop.
The Modern Periodic Table is similar to that of Mendeleev, but contains several improvements. Elements are arranged in order of atomic number instead of atomic mass. This means that elements no longer have to swap places to fit correctly. Many new elements have been discovered and slotted into the spaces left by Mendeleev. Also metals and non-metals are clearly separated. The Modern Periodic Table is shown in Figure 6.1.
Figure 6.1: The Modern Periodic Table

The long form of the periodic table is the commonly used form of the periodic table. The elements in the table are arranged based on their atomic weights, starting from hydrogen (1), helium (2), lithium (3), beryllium (4) and so on. The elements appear in vertical columns and horizontal rows.
The vertical columns in the table are called Groups, numbered I, II, III, IV, V, VI, VII and 0, which is also known as group VIII. Group I contains the elements lithium (L), sodium (Na), rubidium (Rb), caesium (Cs) and francium (Fr). Group II consists of elements starting from sodium (Na) down to radium (Ra). Some of the groups have special names.
- Group I is often called the alkali metals.
- Group II the alkaline earth metals.
- Group VII the halogens.
- Group 0 the noble gases.
The transition metals (or elements) form a separate block in the middle of the periodic table between group II and III. The atoms of these elements have more complicated electron arrangements. Note that the group contains many common metals such as iron (Fe), Nickel (Ni), copper (Cu), and Zinc (Zn). One of the interesting properties of these elements is that they form coloured compounds.
Main features of the Modern Periodic Table
- The elements in the table are placed in order of their atomic numbers instead of their atomic masses.
- There are a total of 18 groups and 7 periods.
- There are 5 blocks of similar elements in the periodic table as shown in figure 6.2.
- The normal (non-transition) elements (groups 1-7) have their outermost shells incomplete, meaning that they can allow additional electrons to enter into their outermost orbital (valency shell). But each of their inner shells is complete.
- The transition metals have their outermost as well as their penultimate (second last) shells incomplete.
- Elements of group 0 (noble gases) have their shells complete. These elements show little reactivity. That is why they wereonce called „inert‟ gases because they are very unreactive; or „rare gases‟ because they were rarely found.
- Gaps left by Mendeleev for undiscovered elements (now occupied by the transition elements and the noble gases) have been filled by the respective elements following their discovery. Man-made elements have also found a place in the periodic table.
- Metals have been clearly separated from non-metals. Metalloids or semi metals (poor metals) have also been included. Metalloids are elements whose properties are intermediate between metals and non-metals. They include boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb) and tellurium (Te). In some publications, germanium and antimony are usually classed as poor metals and the rest as non-metals.

Periodicity
The Concept of Periodicity
Explain the concept of periodicity
Consider the electronic configuration of the first twenty elements of the periodic table shown in the table below.
Table 6.3: Electronic configurations of the first 20 elements

You will notice that elements in the same vertical columns (groups) have the same number of electrons in the outermost shells of their atoms. Because the outer electrons determine the chemical properties of an element, then the elements in each period tend to resemble each other closely in chemical behaviour. For instance, the noble gases, He, Ne and Ar show a chemical inertness which is characterised by the stable outer electron octet or duplet. Due to this reason, the compounds of the noble gases with other elements have not been found.
Attempts to classify elements by arranging them in order of increasing atomic weights shows that the properties of elements were periodic. This means elements with similar or comparable properties appear after a certain specific interval in a given arrangement. The occurrence of successive groups of elements showing strong chemical similarity in this way is called periodicity.
Therefore, periodicity is the repetition of similar chemical properties of elements after a certain specific interval in a given arrangement. The repetition in properties is due to repetition of similar electronic configuration of outermost shells of elements after certain intervals.
General Trends
This refers to change in some properties of elements across the periods and down the groups in the periodic table. These trends become more obvious if we leave aside the noble gases in Group 0. In this case, we shall concentrate our efforts on variations in the most important properties of the elements only. The following is a summary of the change in some properties of elements down the groups and across the periods.
The Change in Properties of Elements Across the Periods
Explain the change in properties of elements across the periods
Atomic and ionic size
The sizes of atoms and ions may be given in terms of atomic radius and ionic radius units respectively. The number of shells an atom or ion posses and the nuclear charge determines the size of an atom or ion. This is how the two properties vary along the period and down the group:
Atomic size
Along the period: Considering the normal elements only, the size of the atoms decrease from left to right across the period. This is because as atomic number increases across the period, the nuclear charge (due to increasing protons) increases and electrons in shells are pulled closer to the nucleus.
Ionic size
- Positive ions (cations):Across the period; The ionic size does not change, i.e. remains the same, as you move across the period from either direction.
- Negative ions (anions):A negative ion is larger compared to the corresponding neutral atom because on forming an ion, one or more electrons are added to the atom. The added electron(s) is/are repelled by the electron(s) already present in the outermost shell, hence leading to an increase in the size of an atom, even though no new shell is formed.Down the group and along the period: Ionic size increases down the group, and along the period, i.e. from left to right.
Atomic radii (singular: radius)
Along the period: In the period, atomic radii decrease from left to right with increase in the atomic number.
Electronegativity
Electronegativity is the tendency of an atom to attract the shared pair of electrons towards itself in a molecule. The electronegativity values of elements in group 0 (inert gases) is zero.
Along the period: Electronegativity increases while moving across the period from left to right in the periodic table.
Metallic character (or electropositivity)
Electropositivity is the tendency of an element to lose the valency electron(s) and donate the same to other elements (usually non-metallic elements). This process occurs during the formation of new substances e.g. molecules and compounds. Literally, such reactions occur between metals and non-metals whereby metals donate electrons and non-metals receive these electrons. So, metals are electropositive elements while non- metals are electronegative elements.
Along the period: Generally, metallic character decreases along the period from left to right.The gradation in metallic properties across the period is as follows: Metals → poor metals → metalloids → non-metals → noble gases
Chemical reactivity
Reactivity is the tendency of an element to lose or gain electrons in a chemical reaction.
Along the period: For metals, the reactivity decreases from left to right in a period while it increases for non-metals.
Ionization Energy or Ionization Potential (I.E or I.P)
This refers to the minimum amount of energy required to remove the most loosely bound electron from an isolated atom or ion in its gaseous state. The smaller the value of ionization energy, the easier it is to remove the electron from the atom.M(g) →M+(g) + e-
Along the period: It increases along the period from left to right with the increase in atomic number.
Electron affinity (Ea):
This is just opposite to I.E. It is defined as the amount of energy released when an extra electron is added to an isolated neutral atom in its gaseous state.
Along the period: The value increases along the period from left to right.
Density and melting point
The density of a substance is the ratio of its mass to its volume, while the melting point is the temperature at which a solid substance turns into liquid at standard atmospheric pressure.
- Density-Across the period: Densities decrease across the period from left to right.
- Meting point-Across the period:Melting points of elements decrease across the period from left to right.
The Change in Properties of Elements Down the Groups
Explain the change in properties of elements down the group
Atomic and ionic size
- Atomic size-Down the group: Atomic size increases as you move down the group.
- Ionic size- Positive ions (cations)-Down the group: On descending the group, the nuclear charge increases and the number of shells increase by one at each step so, the ionic size also increases. A positive ion is smaller than the corresponding neutral atom because on forming the ion, the metal atom loses both the valency electron(s) and the outermost shell. Valency electron(s) refer(s) to the electron(s) in the outer-most shell of an atom. Any further removal of electron(s) from the ion will decrease the ionic size further.Negative ions (anions)-Down the group and along the period: Ionic size increases down the group, and along the period, i.e. from left to right.
Atomic radii (singular: radius)
Atomic radius is the distance from the centre of the nucleus to the outermost shell (valency shell). Down the group: Atomic radii of elements increase down the group with increase in atomic size.
Electronegativity
Down the group: Electronegativity decreases while moving downwards in a group.
Metallic character (or electropositivity)
Down the group: Metallic character (electropositivity) increases down the group
Ionization Energy or Ionization Potential (I.E or I.P)
Down the group: It decreases gradually down the group.
Why is there a decrease in I.E as you go down the group? This is because electrons are held in their shells by their attraction to the positive nucleus, and as you go down the group, the size of the atom increases (increasing atomic radius). So, the outermost electron(s) of an atom gets further and further away from the attraction or pull of the positive nucleus, hence requiring little energy to remove from the atom.
Electron affinity (Ea)
Down the group: The value of electron affinity decreases down the group.
Density and melting point
- Density-Down the group: Densities of elements increase down the group.
- Meting point-Down the group:Melting points of elements decrease down the group as the elements become less metallic in nature.
Electronic Configuration to Locate the Positions of Elements in Periodic Table
Use electronic configuration to locate the positions of elements in periodic table
The modern periodic table is based on electronic configurations of the elements. Look at table 6.3 and study the electronic configurations of the first twenty elements and where they are placed in the periodic table.
Beryllium, magnesium and calcium have two electrons in the outer shell. These elements are in Group 2.
This pattern continues to Group 3, Group 4 and so on. The group number in the periodic table is the same as the number of electrons in the outermost shell. The halogens are the elements in Group 7. Bromine is one of the halogens. How many electrons does each bromine atom have in its outer shell?
As we move down each group, the number of shells increases by one at each step. Each atom of an element has one complete shell than the one above it.
As we move across each period, the outer shell is being filled by one electron at each step. Certain electronic configurations are found to be more stable than others are. The noble gases at the end of each period have full outer shells. They have stable duplet (2 electrons) or octet (8 electrons) in their outermost shells. This makes them more difficult to break up, and this fits well with the fact that they are so unreactive.
The outer electrons of an atom are mainly responsible for the chemical properties of an element. Therefore, elements in the same group will have similar chemical properties.
TOPIC 7
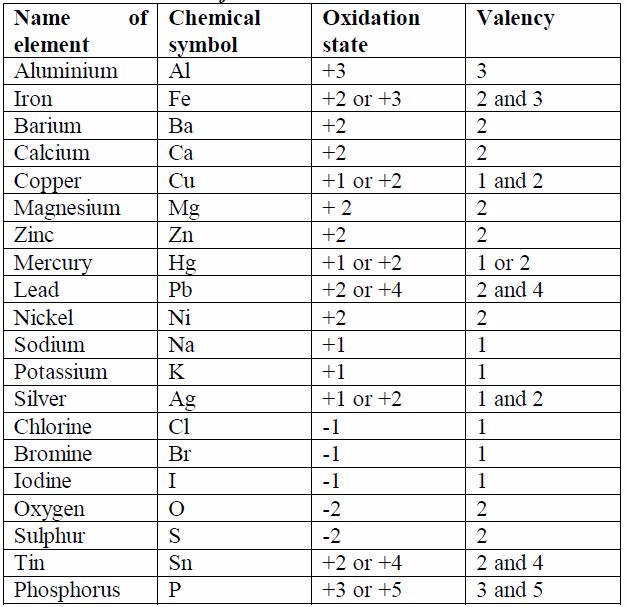

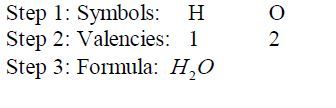
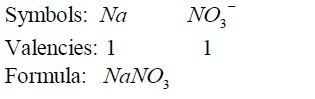

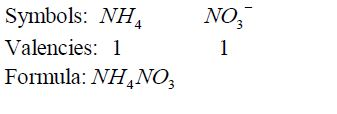
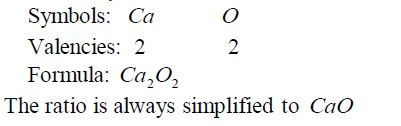
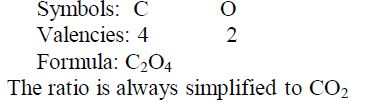
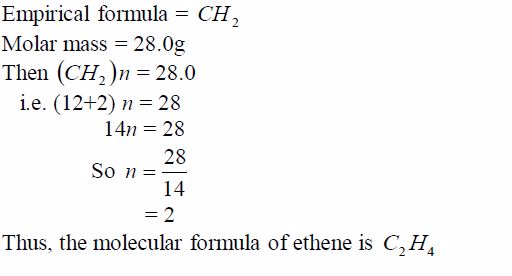

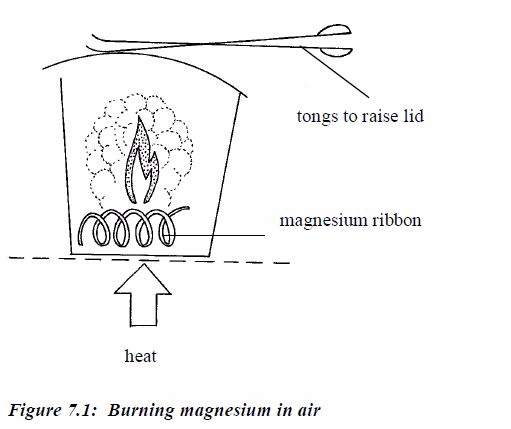
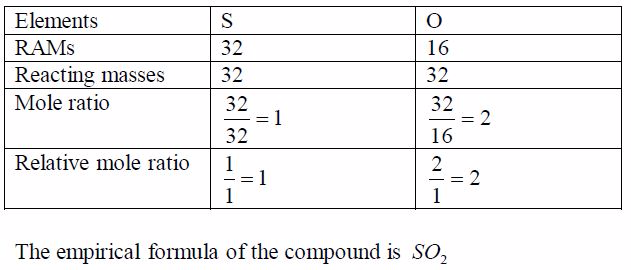
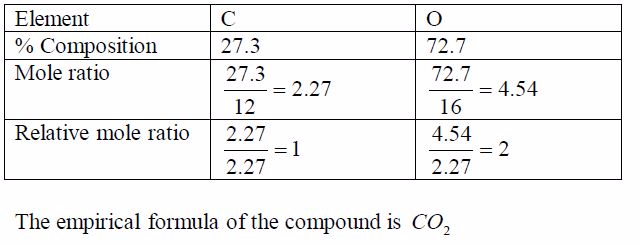
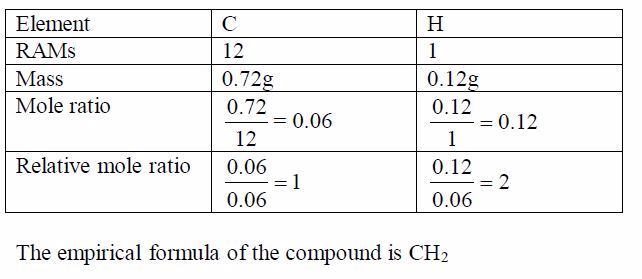
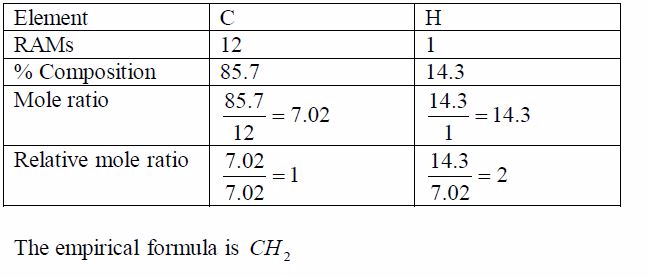

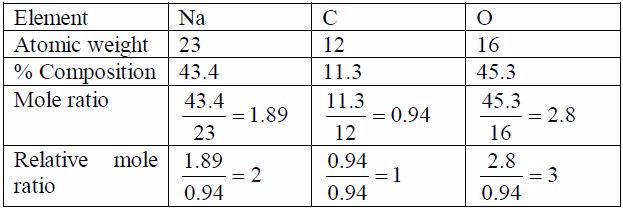
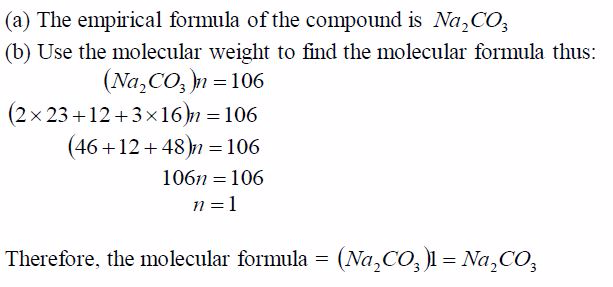
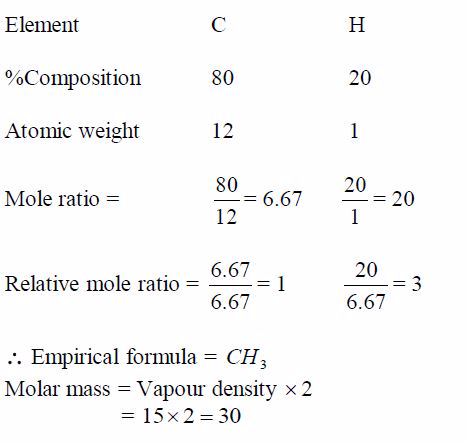
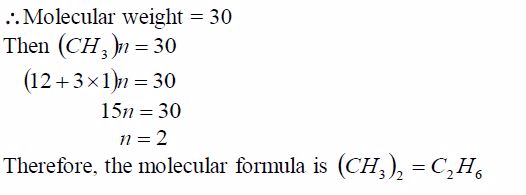
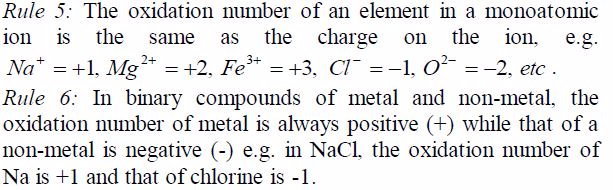
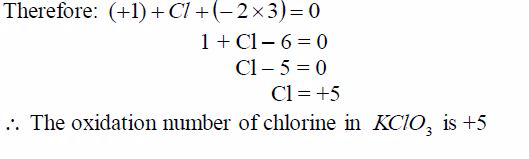
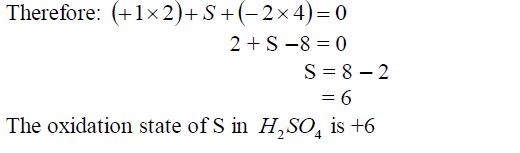
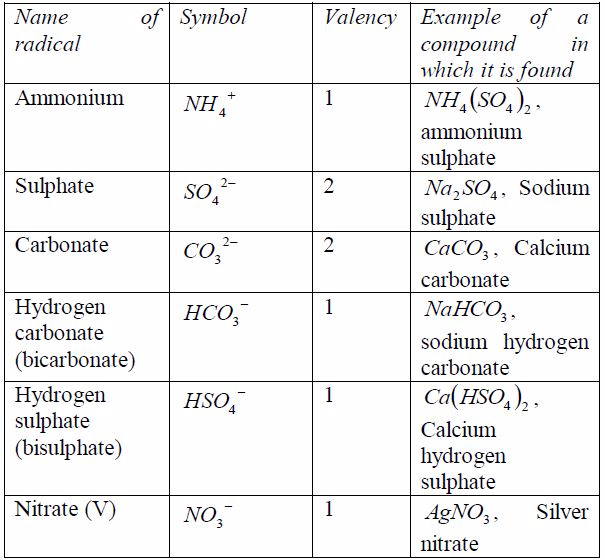
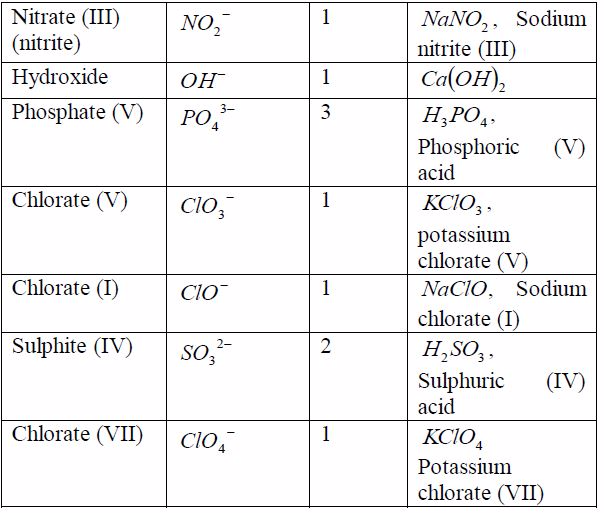

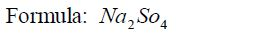
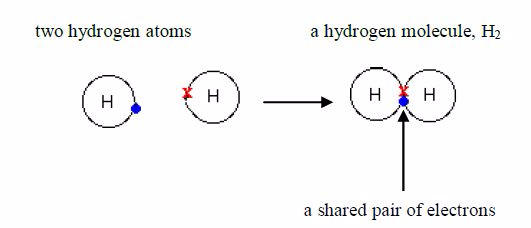
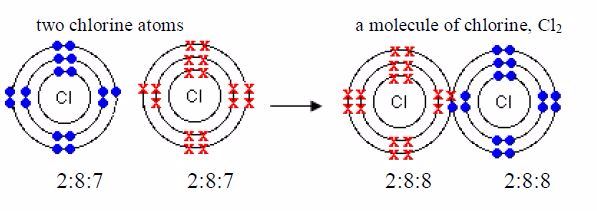
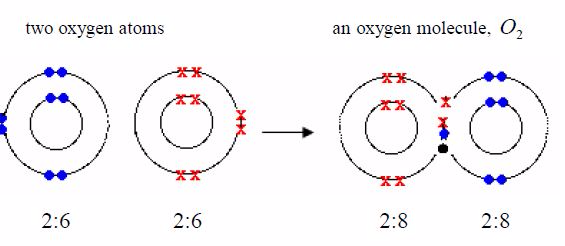
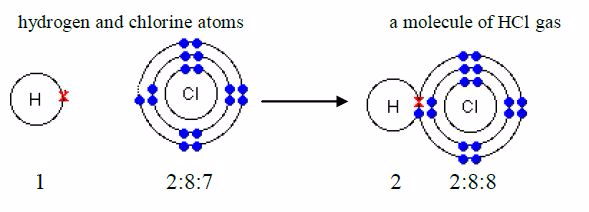
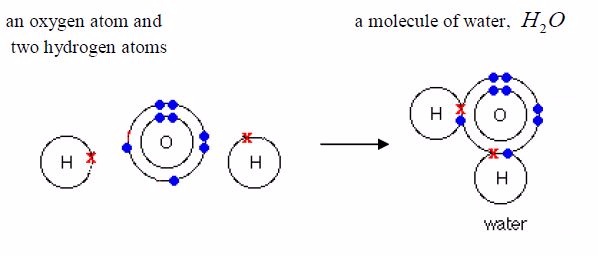
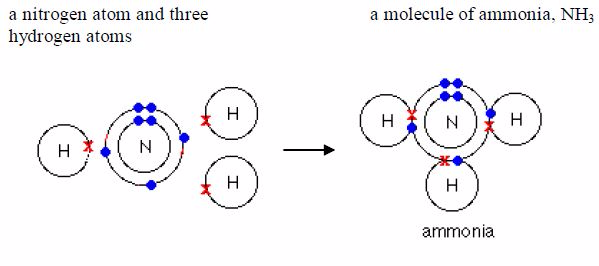
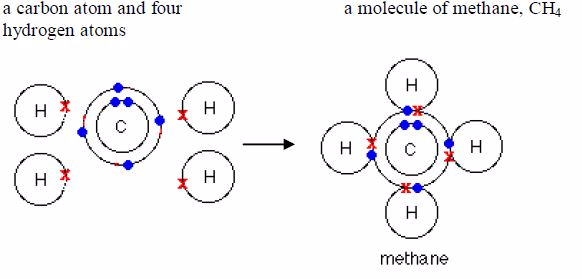
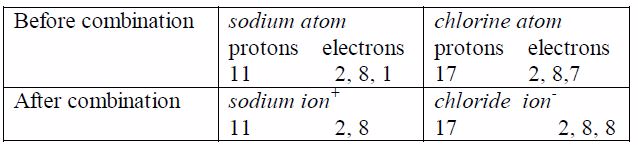
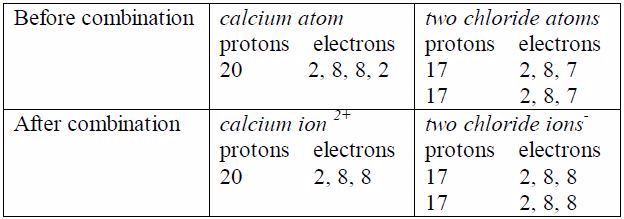
Valence and Chemical Formulae
The Concept of Valence
Explain the concept of valence
Valency is the capacity of an atom to combine with one or more atoms to form a molecule or compound. Valency also refers the number of electrons that an atom can gain, lose or share in forming a chemical bond with another atom. The valency (or combining power) depends on the number of electrons in the outermost orbit (or valency shell) involved in the formation of a chemical bond. The number of electrons in the valency shell is never greater than 7. The outermost electronic configuration is responsible for the variability of the valency.
Some elements exhibit more than one valency, i.e., they have variable valencies. Examples of elements with variable valencies are iron (2 and 3), tin (2 and 4) and copper (1 and 2). The other elements with variable valencies are as shown in table 7.1.
Valency and Oxidation States
There is a strong correlation between valency and oxidation state. The oxidation state of an element equals its valency or charge carried by its ion when an element ionizes in solution. An example of this relation is iron (II) whose oxidation state (or oxidation number) is 2 and its valency is 2. The same case applies to iron (III). Other elements with variable valencies such as copper (I) and copper (II) have oxidation state equal to 1 and 2 respectively. The list continues. You will learn more about oxidation states later.
The valencies of the common transition elements should be memorized. Valencies of the normal elements may be deduced from the group number they occupy in the Periodic Table. The valencies of elements of group I to IV are equal to the group numbers they occupy in the periodic table. The valency of an element in group V to VIII is equal to eight minus the group number. For example, the valency of chlorine which is in group VII is 1, i.e. (8 -7) =1. The valency of oxygen in group VI is 2, i.e. (8-6) =2. Elements in group 0 (or VIII) have zero valency i.e. (8 - 8) = 0.
Table 7.1. Valencies of common metals and non-metals

Simple Formulae of Binary Compounds
Write simple formulae of binary compounds
Chemical Formulae
The Chemical formula is a method of representing the molecule of a compound by using chemical symbols. It is a way of expressing information about the atoms that constitute a particular chemical compound. The chemical formula identifies each constituent element by its chemical symbol and indicates the number of atoms of each element found in each single molecule of that compound.
The symbol for hydrogen atom is H. When two hydrogen atoms join together they form a molecule,H2. The number 2 to the right and below the symbol shows the number of atoms a molecule contains. P4 and S8 represents 4 atoms of phosphorus and 8 atoms of sulphur contained in one molecule of phosphorus and one molecule of sulphur respectively.
While the formula for chlorine molecule isCl2, it cannot be expressed as 2Cl. This is because 2Cl means two atoms of chlorine and not a molecule of chlorine.H 2 O stands for a molecule of water which consists of two hydrogen atoms and one oxygen atom.
H2SO4stands for a molecule of sulphuric acid containing 2atoms of hydrogen, 1 atom of sulphur and 4 atoms of oxygen.CaCO3 stands for a molecule of calcium carbonate containing 1atom of calcium, 1 atom of carbon and 3 atoms of oxygen.Where it deems necessary to show the number of molecules a compound contains, this is achieved by writing the appropriate number before the formula of the compound. A few examples are shown below:
- 2H 2O means two molecules of water
- 3H2 SO4 means three molecules of sulphuric acid
- 5CaCO3 means five molecules of calcium carbonate
It is important to note that the figure appearing before the formula multiplies the whole of it. For example, 3H2SO4 stands for 3 molecules of sulphuric acid containing six atoms of hydrogen, three atoms of sulphur and twelve atoms of oxygen.It is a big mistake to think that the number 3 before the molecule multiplies only the symbol which immediately follows it (that is,H2). This is quite wrong. The 3 multiplies the whole of the formula
Formulae of Binary Compounds
A binary compound is a compound made of only two types of the reacting species, for example, sodium chloride (NaCl),which is made of only sodium and chlorine, is a binary compound. Look at table 7.1. The size of the charge on an ion isa measure of its valency or combining power. You will notice that, ignoring the signs for the charge on ions, the value of the charge on ion is equal to the valency of the atom. You will need to memorise the valencies of these metals as much as possible so as to be able to write the formulae of their compounds correctly.
The following are the rules for writing down the chemicalformulae of chemical substances:
- Metals (or their positively charged ions) must start in theformula, followed by non-metals (or their negatively chargedions).
- Where the formula is to include a radical, the radical must betreated as a single atom and must be bracketed if need be.
- Ammonium ion is to be treated as if it were a metal.
- Positive charges must be equal to negative charges for aneutral molecule or compound.
- Single elements; say Na, K, Si, Ag, etc. should not bebracketed.
- Valencies of metals (or positive ions) should be exchangedand written as subscripts.
- The valency of 1 is simply assumed and not written in theformula.
This is best shown by using some examples. The following procedure must be followed when writing the formulae of binary compounds:
- Write down correct symbols for atoms of elements or ions that make up the compound.
- Write down the valencies of the atoms of the elements.
- Interchange the valencies and write them as subscripts in the final formula of the compound. Remember that the valency of 1 is not expressed in the formula. At this final step, the radicals must be bracketed if necessary.
Study the following examples and make sure you understand how this works:
Write the formula for aluminium oxide:

Write down the formula for water:
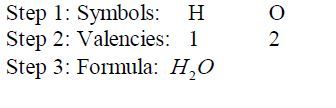
Formula for sodium nitrate:
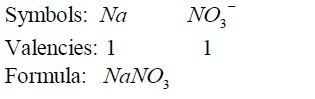
Formula for calcium nitrate:

Formula for ammonium nitrate:
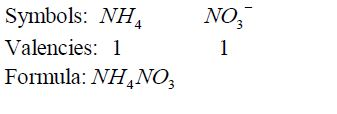
Formula for calcium oxide:
Formula for aluminium sulphate:
Ammonia:
Carbon dioxide:
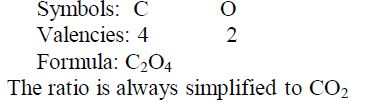
Nomenclature of Inorganic Compounds
The term "nomenclature" refers to "system of naming". The system of naming in use is that recommended by the IUPAC (International Union of Pure and Applied Chemistry). The modern system of naming reveals the type of elements present in a given compound. The old or trivial names have been dropped out.
Some common and important compounds have historical names that do not seem to fit in the system, for example water H O 2 ,ammonia 〈NH3〉, methane , CH4and mineral acids such as sulphuric (VI) acid〈H2SO4〉, nitric (V) acid〈HNO3〉and hydrochloric acid〈HCl〉. Also organic acids such as ethanoic acid (CH3COOH) are also included in this group. These names are trivial but they have been adopted in modern nomenclature.If these exceptions are omitted, there are basic generalizations that are useful:
- If there is a metal in the compound, it must be named first. In this case ammonium ion, NH4¹, is regarded as if it were a metal in the compounds it occurs such as NH4 NO3 , NH Cl 4 , etc.
- For elements with variable valencies such as iron and lead,Roman numerals are included in the name to indicate the valency of the metal or the ion which is present. For example,iron (III) chloride contains Fe3+ while iron (II) chloride contains Fe² . The same case applies to lead (II) and lead (IV)compounds and so on.
- Compounds containing two elements only (binary compounds) have names ending in …..ide; for example sodium chloride (NaCl), calcium bromide (CaBr2), magnesium nitrite( Mg3 N2) , etc. The important exception to this is hydroxides,which contains the hydroxide (OH) ion.
- Compounds containing a poly atomic ion (usually containing oxygen) have names that end with …ate; for example calcium carbonate (CaCO3) , potassium nitrate(KNO3) , magnesium sulphate(MgSO4) , sodium ethanoate (CH3 COONa) , etc.
- The names of some compounds use prefixes to tell you the number of that particular atom in the molecule. This is useful if two elements form more than one compound. For example:carbon monoxide (CO), carbon dioxide (CO2), nitrogen dioxide NO2 dinitrogen tetra oxide N2 O4 , sulphur dioxide SO2 sulphur trioxide SO3 , etc.
The following prefixes indicate the number of atoms in caseslike this: mono – one; di – two; tri – three; tetra – four; pent –five; hex – six; hept – seven; oct – eight; non – nine; and dec –ten.
The Concept of Empirical and Molecular Formulae
Explain the concept of empirical and molecular formulae
The empirical formula is the simplest formula of any compound.It expresses the simplest ratio of all the atoms or ions that makeup a certain compound. For example, the empirical formula ofthe compound with the formula, C2H4is CH2. This means thatthe simplest ratio of (C:H) is 1:2. This ratio also indicates theratio in which carbon and hydrogen atoms combine to form thecompound C2H4.
The molecular formula is the formula which shows the actual number of all atoms present in a given compound. For example,the molecular formula of the above compound is C2H4. This means that two atoms of carbon and four atoms of hydrogen form the compound. Likewise, the molecular formula of water isH2O meaning that the compound is made up of two atoms of hydrogen and one atom of oxygen.
Therefore, the empirical and molecular formulae can each bedefined thus:
The empirical formula of a compound is the simplest formula which shows its composition by mass, and which shows the ratio of the number of the different atoms present in the molecule.
The molecular formula of a compound is the one which showsthe actual number of each kind of atom present in its molecule.
The empirical formula differs from the molecular formula of the same compound since only the molecular formula agrees with the molar mass of the compound.
Information given by Empirical and Molecular Formulae
The formula for water is H 2 O . From this information, you can see that:
- 2 hydrogen atoms combine with 1 oxygen atom to form one molecule of water.
- moles of hydrogen atoms combine with 1 mole of oxygen atoms. Moles can be changed to grams using relative atomic masses (RAMs). So we can write:
- grams of hydrogen combines with 16 grams of oxygen. In the same way:
- 1g of hydrogen combines with 8g of oxygen.
- 4g of hydrogen combines with 32 of oxygen.
The masses of each substance taking part in the reaction are always in the same ratio.Therefore, from the molecular formula of a compound you can tell:
- how many moles of different atoms combine;
- how many grams of the different elements combine;
- the number of each kind of atoms of different elements that combine to make up a compound; and
- the percentage of each atom in a compound based on RAMs of each atom And from the empirical formula you can tell:
- the simplest ratio or proportion of the different atoms that combine to form a compound.
Example 1
the empirical formula of ethane C2H4andpropene C3H6with molar masses 28.0g and 42.0grespectively is CH2(i.e. the same) although the two compounds possess different molecular formulae and masses.
In general, the empirical formula multiplied by a whole number,n, gives the molar mass of the compound. So long as the value of n is known, then the molecular mass can be deduced.For example, suppose the molecular mass of ethene is 28.0g, its molecular formula can be deduced thus:

Similarly, suppose carbon dioxide has a molar mass of 44g and its empirical formula is CO2. Its molecular formula can be determined thus:

The Empirical and Molecular Formulae
Calculate the empirical and molecular formulae
The Empirical Formula
Chemists find the percentage by mass of each element in a compound by experiment. Using this information, it is then possible to find the simplest formula of that compound. To do this we shall also need to know the relative atomic mass of each element present in the compound.
An experiment to find the empirical formula of a compound
To work out the empirical formula you need to know the masses of elements that combine. For example, magnesium combines with oxygen to form magnesium oxide. The masses that combine can be found like this:
Procedure
- Weigh a crucible and lid, empty. Then add a coil of magnesium ribbon and weigh again.
- Heat the crucible. Raise the lid carefully at intervals to let oxygen in. The magnesium ribbon burns brightly.
- When burning is complete, let the crucible cool (still with its lid on). Then, weigh again. The increase in mass is due to oxygen.
Results
Here are sample results and the calculation:
- Mass of crucible + lid = 25.2 g
- Mass of crucible + lid + magnesium = 27.6g
- Mass of crucible + lid + magnesium oxide = 29.2g
- Mass of magnesium = 27.6g – 25.2g = 2.4g
- Mass of magnesium oxide = 29.2g – 25.2g = 4.0g
- Mass of oxygen, therefore = 4.0g – 2.4g =1.6g
Therefore, 2.4g of magnesium combines with 1.6g of oxygen.The RAMs are Mg = 24, O = 16. Changing masses to moles:24/2.4moles of magnesium atoms combines with1.6/16 moles of oxygen atoms
0.1 moles magnesium combines with 0.1moles of oxygen atoms.So the atoms combine in a ratio of 0.1:0.1 or simply 1:1The empirical formula of magnesium oxide = MgO

Sometimes the empirical formula can be determined from the provided percentage composition or weight of atoms of the elements that constitute a compound.
Example 2
An experiment shows that 32g of sulphur combines with 32g ofoxygen to form the compound sulphur dioxide. What is itsempirical formula?
Solution
Divide each mass by the RAM of the respective element. This gives the ratio of different numbers of atoms of each element that make up the compound. Again, divide each of these ratios by the smallest to get the whole number ratio. This gives the simplest ratio of the constituent elements possible. Sometimes you may not get a whole number ratio. If this happens, round off the ratio to the nearest whole number. Finally, write down the formula using the obtained ratio of the elements. Study the following table and make sure you understand the procedure:

Example 3
An oxide of carbon contains 27.3% carbon. Find the empirical formula of the oxide.Think big!What other element is present in the oxide of carbon?How do you know that the percentage of this other element is72.7%?
Solution
In order to work out the simplest formula we divide each percentage by the relative atomic mass of each element. This allows a comparison of the different numbers of atoms of each element that are present. We get a ratio of each element with respect to each other as worked out in the table below. To get a whole number ratio, we again, divide each of these ratios by the smallest.The result shows the simplest ratio of atoms, in this case one carbon atom to two oxygen atoms. The simplest ratio, therefore,is CO2 .

Example 4
A compound X is a hydrocarbon. It contains only carbon and hydrogen atoms. 0.84g of X was completely burned in air. This produced 2.64g of carbon dioxide CO2 and 1.08g of water H2 O. Find the empirical formula of X.
Solution
- In CO2 ,12/44of the mass is carbon
- All the carbon came from X
So 2.64g of CO2 contains 2.64g〈12/44〉*2.64g= 0.72g of carbon and,therefore, 0.12g of hydrogen (0.84g – 0.72g = 0.12g)Since we have deduced the weights of the respective elements in the compound, we can now work out the empirical formula as usual:

The Molecular Formula
The molecular formula is more useful than the empirical formula because it gives more information. For some molecular compounds, both formulae are the same. For others they are different.
To find the molecular formula of an unknown compound youneed to know:
- the empirical formula; and
- the molecular weight of the compound.
Once you have these two pieces of information you can now work out the molecular formula.
Example 5
A compound contains 85.7% carbon and 14.3% hydrogen. Itsmolecular weight is 28. What is its molecular formula?
Solution
Step 1: Find the empirical formula of the compound:

Step 2: Find the molecular formula using the relation 2 CH n =Molecular formula:

Example 6
A compound contains 43.4% sodium, 11.3% carbon and 45.3%oxygen.
(a) Find the empirical formula of the compound.(b) If its molecular weight is 106, calculate its molecularformula.
Solution


In some cases, the molecular weights are given while in others the vapour density is given. To get the molecular weight,multiply the vapour density by two, e.g.:Molecular weight = Vapour density 2
Example 7
A hydrocarbon has a vapour density of 15, and it contains 20%by mass of hydrogen. Calculate the formula of the hydrocarbon.
Solution
If H = 20%, then C = 80%
Use these values to find the relative number of moles of atoms present:

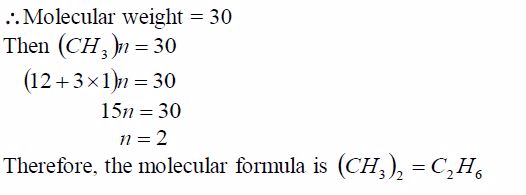
Oxidation State
The Concept of Oxidation State
Explain the concept of oxidation state
Oxidation state (or oxidation number) gives information about the number of electrons an element has lost, gained or shared on forming a compound. An element gains, loses or shares electron(s) only when it reacts to form a compound. An element in a free state has an oxidation state of zero.
There is a close correlation between valency and oxidation state.Oxidation state of an atom in a compound is normally assigned relative to the other elements in that particular compound. So you find that the oxidation state of a particular atom may change depending on a compound in which it is. For instance, the oxidation state of sulphur in SO2 is +4, whereas in SO3 is +6.
Rules for assigning oxidation numbers
Rule 1: An oxidation number of an element in a free(uncombined) state is zero e.g. Na, Li, K, Zn, etc.
Rule 2: Some elements nearly always have the same oxidation number in their compounds. These elements can be used as reference points in assigning oxidation numbers to the other elements. For example:
- Fluorine in all its compounds shows an oxidation number of -1.
- Chlorine in all its compounds has an oxidation number of -1 except when combined with fluorine or oxygen.
- Oxygen in all its compounds has an oxidation state of -2except in peroxides where it has an oxidation state of -1and oxygen difluoride (OF2) in which it has an oxidation state of +2.
- Hydrogen in all its compounds exhibits an oxidation state of +1 except in metal hydroxides where it shows an oxidation state of -1.
- Potassium in all its compounds exhibits an oxidation state of +1.
Rule 3: The algebraic sum of oxidation numbers of all elementsin a radical e.g. SO4 is equal to the charge possessed by theradical e.g. for the sulphate radical ( SO4 ) it is -2.
Rule 4: The algebraic sum of oxidation numbers in a neutralmolecule (or compound) is always equal to zero.
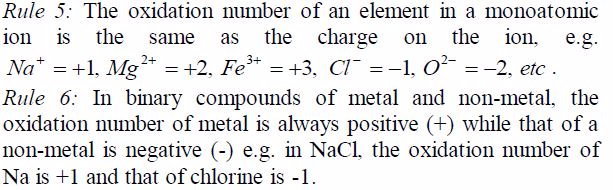
Calculations of oxidation numbers
Example 8
Find the oxidation state of chlorine in the compound, KClO3
solution
The oxidation number of potassium, K = +1 and that of oxygen,O = —2. But since there are 3 oxygen atoms, we have 2*3 =—6.KClO3 is a neutral compound, so the sum of oxidation numbersof all elements in the compound is zero.
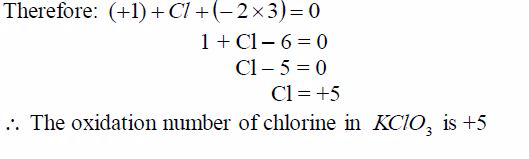
Example 9
Find the oxidation state of sulphur in a molecule, H2 SO4
solution
The oxidation state of oxygen = -2The oxidation state of hydrogen = +1The sum of oxidation number of all elements in H2 SO4
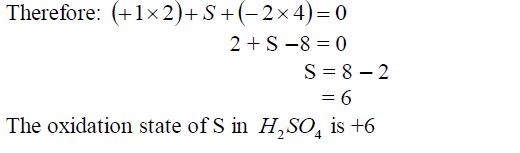
Difference between Oxidation State and Valence
Differentiate oxidation state and valence
Valence electrons are the electrons that participate in forming chemical bonds. For example, lets look at the element carbon. Carbon has a total of 6 electrons (you can tell this by looking at the periodic table). However, 2 of those electrons are in the core of the atom ( in the 1s orbital). The remaining 4 electrons are in the outer 2s and 2p orbitals. Since these 4 electrons are in the outer shell, they can participate in bonding. Therefore since there's 4 electrons that carbon can share, we say that carbon has a valency (or as you call it a valency number) of 4.
Oxidation state is a number used to designate how oxidized an atom is in a compound or molecule. It is the hypothetical charge an atom would have if all of its bonds were completely ionic (rather than covalent). Really oxidation state is a book-keeping formalism that allows us to track what is being oxidized or reduced in a chemical reaction by comparing the oxidation states of the reactants to those of the products.Hope that helps.
Radicals
The Concept of Radicals
Explain the concept of radicals
A radical is a group of elements which behaves like a singleatom in forming compounds. Radicals do not exist alone. Theyare always found combined with metals. The valency of theradical is equal to the charge on it. Table 7.6 shows the valenciesof different radicals. Examples of compounds in which theradicals can be found are also included in the table. All radicalsare charged. The only common positively charged radical isammonium radical. All these radicals except ammonium radical,which is positively charged, can combine with hydrogen or othermetals.
Table 7.6: Some radicals and their valencies


As you have seen in the examples in the table above and in theprevious section, when writing the formula of any substance,you have to take into account the valencies of the reactingelements or radicals.
Example 10
Write the chemical formula of sodium sulphate
Solution
Step 1: Identify the elements or radicals in the compound:Sodium sulphate is a compound whose every molecule is madeup of a sodium metal and a sulphate radical.
Step 2: Write the symbols of the element and the radical; andtheir valencies:

Step 3: Interchange the valencies of the metal and radical andthen write down the formula.
Chemical Formulae of Common Compounds
Write chemical formulae of common compounds
As you have seen in the examples in the table above and in theprevious section, when writing the formula of any substance,you have to take into account the valencies of the reactingelements or radicals. the example is above.
Covalent Bonding
The Concept of Covalent Bonding
Explain the concept of covalent bonding
Covalent bonding is a type of bonding which involves equalsharing of electrons between two or more atoms participating inbond formation. It is, generally, the property of non-metals to form covalent bonds. In a normal covalent bond, only electronsin the outermost shell of an atom are available for bondformation. Atoms share electrons so as to form a stable electronstructure of the nearby noble gas atom. Consider the bonding inthe following atoms:
Hydrogen
Hydrogen atom possesses only one electron. Its shell holds amaximum of 2 electrons, so it is not full. When two hydrogenatoms make a bond, each one donates one electron to the sharedpair to form a stable helium structure.

Because atoms share electrons, there is a strong force ofattraction between them, holding them together. This force iscalled a covalent bond. The bonded atoms form a molecule.
Hydrogen gas is made up of hydrogen molecules and, for thisreason; it is called a molecular substance. Its formula is 2 HSeveral other non-metals are also molecular. For exampleChlorine, Cl2 , nitrogen, N2 iodine, I2 , oxygen O2 , sulphur,S8 , phosphorus, P4 , etc.
Chlorine
One molecule of chlorine is made up of two chlorine atoms.Each chlorine atom has an electronic configuration of 2:8:7. Itsconfiguration is only one electron less than the stable structurea shared pair of electrons of argon, 2,8,8. Each of the two chlorine atoms contributes oneelectron to the shared pair during chemical combination.

Oxygen
The formula for oxygen is O2 . So each molecule must containtwo atoms. The electronic structure of oxygen is 2:6. Eachoxygen atom has only six outer electrons. So it needs two moreelectrons to reach a stable neon structure, 2:8. Therefore, eachatom contributes two electrons to be shared.

Hydrogen chloride
The formula for hydrogen chloride is HCl. The electronicstructure for hydrogen atom is 1 and that for chlorine atom is2:8:7. An atom of hydrogen has 1 electron in its shell and achlorine atom has 7 outer electrons. To form a stable dupletstructure of helium, 2, the outer shell of hydrogen atom mustreceive one more electron and in order to form a stable argon structure, 2:8:8, the outer shell of chlorine atom must receiveone more electron. So, each atom must contribute one electronfor sharing.

In the above four examples, each hydrogen atom acquire thehelium structure (duplet), each chlorine atom achieves the argonconfiguration 2:8:8 (octet) and each oxygen atom acquires theneon configuration, 2:8 (octet).
It is possible to form multiple bonds between two non-metalatoms. When two electrons are shared between two atoms, onefrom each atom, we represent them by a single line, e.g. Cl-Cl.When four electrons are shared such that each atom contributestwo electrons, we may represent the double bond formed by twolines, e.g. O=O. Likewise, when six electrons are shared suchthat each atom contributes three electrons, we may represent thetriple bond by three lines, N≡N.
Covalent Compounds
You have seen that several non-metal elements exist asmolecules. A huge number of compounds also exist asmolecules. In a molecular compound, atoms of differentelements share electrons with each other. These compounds areoften called electrovalent compounds because of the covalentbond in them. Water, ammonia and methane are examples.Remember it was pointed early that only the electrons in theoutermost shells take part in bonding. As you can see in the examples below, only electrons in the outermost shells areinvolved in bond formation. Electrons in the inner shells do notnormally take part in bonding.
Water: Its formula is H O 2 . Each oxygen atom shares electronswith two hydrogen atoms.

Ammonia: Its formula is 3 NH . Each nitrogen atom shareselectrons with three hydrogen atoms.

Methane: Its formula is CH4. Each nitrogen atom shareselectrons with four hydrogen atoms.

The Properties of Covalent Bonding
State the properties of covalent bonding
Properties of covalent compounds
- They are often liquids and gases at room temperature.
- They have low melting and boiling points (low heats of fusion and vapourization).
- They are usually soluble in organic solvents such as ethanol, ether, benzene, or carbon disulphide (very few are soluble in water).
- They do not conduct electricity because they contain no ions and so are non-electrolytes. Electrovalent compounds consist of molecules.
Electrovalent Bonding
The Concept of Electrovalent Bonding
Explain the concept of electrovalent bonding
This type of bonding occurs when an atom transfers electron(s) from its outermost shell to the outermost shell of another atom, usually a non-metal. In this type of bonding, an atom of a metallic element or group loses its valency electrons from its outermost shell. The lost electrons pass over to the outer shells of an atom with which the metal is combining. By so doing, the metal will become positively charged due to excess proton(s) left on the nucleus while the non-metal will become negatively charged due to extra electron(s) it has received. The particles are then known as ions. The positive ions are called cations while the negative ions are called anions.
Metal atoms lose electrons to attain the electron configuration ofthe nearest noble gas, while the non-metal atoms gain electronsto attain the electron configuration of the nearest noble gas. Thismeans that an electron octet is left behind in the metal andcreated in the non-metal.
Let us consider the formation of the following compounds froma combination of metallic and non-metallic atoms:
Example 1: Sodium chloride

Both ions now posses stable outer octets, like a noble gas.As you have seen, no molecule of sodium chloride is formed.Cations ( Na ) and anions Cl attract one another and arrangethemselves into a rigid, solid shape called a crystal, but theyremain quite separate. The combination can be expressed only inionic form as NaCl
The ionic compound is thus a cluster of ions in which a positiveion is surrounded spatially by a number of negative ions, while anumber of positive ions similarly surround each negative ion.The force holding together the oppositely charged ions is calledelectrostatic force and hence the name electrovalent bond.

Example 2: Calcium chloride
In the calcium ion, the two excess nuclear protons produce adouble positive charge, and the two electrons released from theouter shell of calcium atom are equally shared between the twochloride atoms. In each chloride ion, the excess electronproduces a single negative charge that is Ca 2Cl

Activity 1
Assignment
Magnesium oxide is an electrovalent compound just like calcium oxide and sodium chloride. Show how the magnesium atom combines with oxygen atom to form the oxide, clearly indicating the process of electron loss and gain
Properties of Electrovalent Bonding
State properties of electrovalent bonding
Properties of electrovalent (ionic) compounds
- Ionic compounds are generally crystalline solids at room temperature.
- They have high melting and boiling points (also high heats of fusion and vapourization)
- They are generally soluble in water but insoluble in organic solvents such as benzene, alcohol and ether.
- They conduct electricity when molten or when dissolved in water (not when solid).





No comments