TOPIC 1: CHEMICAL EQUATIONS
A chemical equation is a representation of a chemical reaction with the help of symbols and formulae of the substances involved in the reaction. It is a chemical shorthand for representing the reacting substance or substances combining (the reactants) and the substance or substances formed as a result of the reaction (the products).
Molecular Equations
A Molecular equation is the one which shows the reactants combining and the products formed, in their elemental or molecular forms in a chemical reaction. An example of a molecular equation is the reaction between sodium and water to produce sodium hydroxide solution and hydrogen gas:
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
In this context, sodium (in elemental form) reacts with water (in molecular form) to produce sodium hydroxide (in molecular form) and hydrogen gas (in molecular form).
Word Equations for given Chemical Reactions
Write word equations for given chemical reactions
A word equation is a short form of expressing a chemical reaction by word. Chemical reactions can be summarized by word equations that show all the reactants and the products. This type of equation links together the names of the reactants and the products. For examples, the burning of magnesium in air to produce magnesium oxide can be represented by the following word equation:
Magnesium + Oxygen → Magnesium oxide
Another example is the reaction between sodium and chlorine to give sodium chloride (common salt)
Sodium + Chlorine → Sodium chloride
Equations like these sometimes give us some information about the products formed when different substances are reacted together. But equations can be made even more useful by writing them using chemical symbols and formulae.
Any method for representing a chemical reaction must meet basic certain requirements. These are:
- the chemical nature of the reactants as well as those of the products must be clear. The reactants can be in solid, gaseous, liquid or aqueous forms.
- the mole ratios in which the products are combined and the products are formed must be deducible. This means that atoms of the reactants and the products must be balanced.
- the direction of the reaction must be established. This means that it should be clearly shown which substances are the reactants and which ones are the products. This is normally done by separating the reactants from the products by an arrow. The arrow normally points from the reactants to the products.
Consider the reaction between potassium and water:
2K(s) + 2H2O (l) → 2KOH (aq) + H2 (g).
In this reaction, the three requirements have been met:
- The chemical nature of the reactants [potassium (solid); water (liquid)] and the products [potassium hydroxide (aqueous); hydrogen (gas)] has been shown.
- The mole ratios of the reactants and products are clearly shown: 2 moles of potassium combines with 2 moles of2water to produce 2 moles of potassium hydroxide and one mole of hydrogen gas.
- The reactants (potassium and water) and the products (potassium hydroxide and hydrogen) are separated by an arrow (→) which also indicates the direction of the reaction.
HOW TO PREDICT REACTION PRODUCTS
To predict the reaction products precisely, one needs to take into account the type of reaction occurring. Once you identify the type of reaction that is going to take place, then you will be in a position of telling what possible products of reaction would be. A chemical reaction is said to have taken place when two or more chemical substances called reactants are converted into very different chemical substances called products.
There are a few ways to predict the reaction products. Firstly, when the reactants are mixed and then isolated, products can be identified. Prediction can also be made when elements from the same group in the Periodic Table show similar reactions. Finally, chemical reactions can be classified into different categories such as combination (or synthesis), decomposition, displacement, precipitation, and redox reactions as described in details below:
Types of Chemical Reactions
When a chemical reaction occurs, it is obvious that the changes have taken place. However, under ordinary conditions it is not easy to see how a reaction goes on. The neutralization of an acid solution with an alkali produces no change that you can see. However, reaction has happened. The temperature of the mixture increases and the new substances have formed which can be separated and purified. Ideally, we can tell whether a reaction has taken place if one or more of the following changes are observed:
(a) heat change has taken place and can be detected by the change in temperature of the products; (b) a precipitate is formed; (c) there is a change in state of the reactants, i.e. gas, liquid; solid; (d) a colour change has occurred; or (e) a gas is evolved and can be identified by its colour, smell or by effervescence.
- heat change has taken place and can be detected by the change in temperature of the products;
- a precipitate is formed;
- there is a change in state of the reactants, i.e. gas, liquid; solid;
- a colour change has occurred; or
- a gas is evolved and can be identified by its colour, smell or by effervescence.
There are very many different chemical reactions. To make it easy to study about these reactions, it is useful to try to group certain types of reactions together. They may be grouped according to certain types of phenomena which accompany them. They can further be subdivided into categories of reactions, each of which has its unique characteristics. Some types of chemical reactions are discussed below:
Combination or synthesis (A + B → C)
Synthesis reaction occurs when two or more simple substances (elements or compounds) are combined to form one new and more complex substance. The general form of a synthesis reaction is:
element or compound + element or compound→ compound.
The reaction between iron and sulphur to form iron (II) sulphide is the best example for this kind of reaction. Iron combines directly with sulphur to form iron (II) sulphide:
Fe(s) + S(s) → FeS(s)
Another example is the reaction between hydrogen and oxygen to form water:
Hydrogen + Oxygen → Water
Decomposition (A →B + C)
Decomposition occurs when one compound breaks down into simpler substances. All decomposition reactions have one thing in common: There is only one reactant and it breaks down into two or more simpler products. Decomposition can be brought about by heat, light, electricity and even enzymes or catalysts.
Decomposition by heat
Decomposition caused by heat is termed as thermal decomposition. An example is the decomposition of calcium carbonate (limestone) which breaks down into calcium oxide(quicklime) and carbon dioxide gas when heated.
Calcium carbonate → Calcium oxide + Carbon dioxide
Formula Equations Using Chemical Symbols
Write formula equations using chemical symbols
Essentially, chemical reactions can be expressed in two forms. The chemical reaction can be expressed either as a word equation or as a formula (or symbolic) equation. We have already seen how chemical equations can be represented by words (word equation). The formula equation makes use of chemical symbols and formulae to represent a chemical reaction. An example is the reaction between iron and sulphur to form iron (II) sulphide: Fe + S → FeS
Steps for writing a chemical equation
These are the steps to follows when writing a chemical equation:
- State the reaction equation in words, for example, carbon reacts with oxygen to form carbon dioxide.
- Write the complete word equation using an arrow to separate the reactants from the products: Carbon + Oxygen → Carbon dioxide. Conventionally, the reactants are placed on the left-hand side and the products on the right-hand side of the equation. An arrow from left to right indicates that the reaction proceeds from reactants to products as shown.
- Change the words into the correct symbols and formulae of the reactants and products: C + O2 → CO2
- Balance the number of each type of atoms on each side of the equation.It is important to make sure that there is equal number of each kind of atom on the left of a chemical equation as on the right in order for your equation to comply with the Law of Conservation of Mass (or Indestructibility of Matter): Matter can neither be created nor destroyed in the course of a chemical reaction. This means that the total mass of all products of a chemical reaction is equal to the total mass of all reactants. All atoms appearing on the left-hand side must also be presented on the right-hand side. No atom should appear as a product if it is not present as a reactant.
- Add the state symbols: Reactants and products may be solids, liquids, gases or solutions. You show their state by adding state symbols to the equation. The state symbol are, (s) for solid, (l) for liquid, (g) for gas and (aq) for aqueous solution (solution in water). For the two reactions above, the equations with the state symbols are: Fe(s) + S(s) → FeS(s); C(s) + O2(g) → CO2(g) All state symbols must be bracketed and placed as subscripts after the reactant(s) and product(s).
Balancing Chemical Equations
Balance chemical equations
A balanced chemical equation has an equal number of atoms of different elements of the reactants and the products on both sides of the equation. A balanced equation gives us more information about a reaction than we get from a simple word equation.
Below is a step-by-step approach to working out the balanced equation for the reaction:
- Write the chemical equation for the reaction with the correct symbols and formulae of the reactant(s) and the product(s).
- Identify different atoms of the different elements of the reactant(s) and the product(s).
- Check whether these different atoms are equal on both sides of the equation. Some atoms may balance each other directly.
- Balance the atoms on each sides of the equation by Hit and Trial Method.
- Add state symbols.
Example 1
The reaction between hydrogen and oxygen to produce water:
Hydrogen + Oxygen → Water
H2 + O2 → H2O (not balanced)
The atoms involved in the reaction are hydrogen and oxygen. It is these atoms that we are going to balance. The atoms must be equal on both sides of the reaction equation. There are two hydrogen atoms on each side of the equation. But, as you can see there are two oxygen atoms on the left-hand side (LHS) of the equation and only one oxygen atom on the right-hand side (RHS). To balance oxygen atoms, we write 2 before water.
H2 + O2 → 2H2O (not balanced yet)
By introducing 2 before water, another problem has been created. Now we have 4 hydrogen atoms on the RHS but only 2 hydrogen atoms on the LHS. To equalize the number of hydrogen atoms we write 2 before hydrogen on the LHS.
2H2 + O2 → 2H2O (balanced).
You can still check to find out whether the atoms are balanced or not. Now look at the number of atoms on each side of the equation:
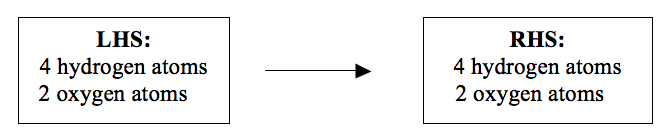
Now, the number of hydrogen and oxygen atoms is the same on both sides of the equation. This is because the atoms do not disappear during a reaction. They are neither created nor destroyed. They obey the Law of Conservation of Mass. When the numbers of different atoms are the same on the both sides, an equation is said to be balanced. Once the equation is balanced you can now add the state symbols.
2H2(g) + O2(g) → 2H2O(l)
This gives a standard and an acceptable chemical equation.
An equation which is not balanced is not correct. An unbalanced equation implies that the atoms have been created or destroyed. It is therefore, wrong and calculations based on it are certainly unreliable.
Remember that we cannot change the formulae of the substances involved in the reaction. These are fixed by the bonding in the substance itself. For instance, in attempt to balance the number of oxygen in water, H2O, we cannot write H2O2. We can only put a multiplying numbers before symbols and formulae, e.g. 2H2O.
Example 2
Hydrogen burns in oxygen to form water. The equation for the reaction is:
2H2(g) + O2(g) →2H2O(l)
- How much oxygen is needed to burn 1g of hydrogen?
- How much water is formed when 5g of hydrogen is completely burned in oxygen? (Atomic weights: H = 1, O = 16)
Solution:
a. Reaction equation:2H2(g)+ O2(g)→2H2O(l)
Atoms present: H : O
Molecular weights: 4 : 32
Reacting weights: 1g : Xg
The weight, X, of oxygen = 1×32⁄4= 8g
So, 1g of hydrogen needs 8g of oxygen
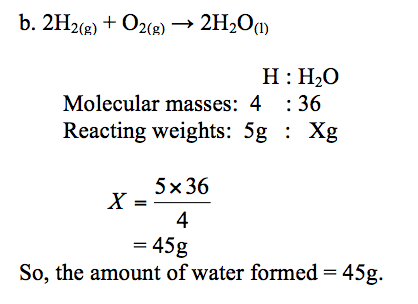
Ionic Equations
The Different Between Molecular Equations and Ionic Equations
Differentiate between molecular equations and ionic equations
Ionic equations are equations in which the reacting substances are represented in ionic forms after the elimination of spectator ions. In other words, ionic equations are those equations represented in such a way that spectator ions are not included in the final equation. Spectator ions refer to those ions, which do not change during the reaction i.e. they do not take part in a chemical reaction.
In order to be able to derive an ionic equation from a molecular equation, one must be acquainted with the solubility rules as outlined below:
- All sodium, potassium and ammonium salts are soluble.
- All nitrates, chlorates and acetates are soluble.
- All binary compounds of the halogens (other than F) with metals are soluble, except those of silver, copper, lead and mercury (lead halides are soluble in hot water).
- All sulphates are soluble except those of silver, lead, mercury (I), barium, strontium and calcium.
- All carbonates, sulphites and phosphates are insoluble except those of ammonium and alkali metal (Group I) cations.
- All hydroxides are insoluble except those of ammonium, barium and alkali metal (Group I) cations.
- All sulphides are insoluble except those of ammonium, alkali metal (Group I) cations and alkali earth metal (Group II) cations.
- All oxides are insoluble except those of calcium, barium and alkali metal (Group 1) cations; these soluble ones actually react with the water (hydrolyse) to form hydroxides.
Balanced Ionic Equations
Write balanced ionic equations
Steps for writing balanced ionic equations
- Write a balanced formula equation for the reaction.
- Split all soluble reactants and products into individual ions, clearly indicating their state symbols. Remember that substances that exists as molecules such as water, gasses and concentrated mineral acids, precipitates and neutral atoms do not consist of ions and hence do not ionize in water.
- Cancel out all the ions which appear on both sides of the equation (spectator ions). These are the ions which remain unchanged in the reaction.
- Re-write the remaining ions. This is the net ionic equation for that reaction.
Example 3
Consider the reaction for neutralization of hydrochloric acid with sodium hydroxide.
- Step 1: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
- Step 2:H+(aq)+Cl-(aq)+Na+(aq)+OH-(aq)→ Na+(aq)+Cl-(aq) + H2O(l)
- Step 3: :H+(aq)+Cl-(aq)+Na+(aq)+OH- (aq)→ Na+(aq)+Cl-(aq) + H2O(l)
- Step 4: H+(aq)+ OH-(aq) → H2O(l)
TOPIC 2: HARDNESS OF WATER
The Concept of Hardness of Water
The Concept of Hardness of Water
Explain the concept of hardness of water
As water flows over the land, it dissolves many mineral substances. The dissolved minerals are deposited together with water in rivers, lakes and oceans. Water is said to be hard if it contains some specific type of dissolved minerals. It is important to note that not all dissolved salts make water hard.
As you learned early, water is treated in water purification plants before being piped to your home. The treatment removes only the insoluble particles and kills bacteria. So the water still is not pure. It contains natural compounds dissolved from rocks and soil. It may also contain traces of chemicals dumped from homes, farms and factories.
Water obtained from an area where the rocks contains chalk, limestone, dolomite or gypsum, contains dissolved calcium and magnesium sulphates and hydrogencarbonates. These salts make the water hard.
One can distinguish between hard and soft water when washing with soap. Hard water does not form lather easily. Instead, it forms a precipitate or scum. It requires much soap to react with all the dissolved minerals before enough lather is formed. Therefore, hard water wastes soap during washing.
When soap is used with hard water a “scum” forms on the surface. This is a result of a precipitation reaction between calcium and/or magnesium ions and soap. Soaps are the sodium or potassium salts of long-chain organic acids. Soaps are made from animal fats by treatment with alkali (NaOH or KOH). Ordinary washing soap is a compound of stearic acid, C17H35COOH. The nature of such soaps is the salt, sodium stearate, C17H35COONa+. Sodium stearate is soluble in water but calcium stearate is not.
When soap is mixed with hard water, the calcium or magnesium salts in the hard water react with soap and precipitates as scum. The nature of scum is either calcium stearate or magnesium stearate:

Soap will not form any lather with water until all the calcium and magnesium ions have been precipitated. Hard water, therefore, wastes soap. This means that more soap may be used for an efficient washing. The amount of soap needed to just produce froth can be used to estimate the hardness of water.
The problem of scum formation only occurs with soaps. Soapless detergents do not produce scum. The trade names for some soapy detergents sold in Tanzania include Komoa, Kuku, Taifa, Mbuni, Mshindi, Changu, Jamaa and several other bar soaps. The trade names for some soapless detergents include Omo, Foma, Tesa, Toss, Dynamo, Swan, etc.
Causes of Permanent and Temporary Hardness in Water
State causes of permanent and temporary hardness in water
Water is generally said to be hard if it contains soluble salts of calcium and magnesium. The salts are calcium and magnesium sulphates and hydrogencarbonates. Hardness of water is caused by higher than usual levels of calcium (Ca2+) and magnesium (Mg2+) ions in water.
As rain water passes through the atmosphere, it dissolves carbondioxide to form a weak carbonic acid.

As this solution passes over and through rocks containing chalk, limestone or dolomite, the rainwater very slowly dissolves them:
H2CO3(aq) + CaCO3(s) → Ca(HCO3)2 (aq)
The calcium hydrogencarbonate formed is soluble in water and is responsible for the presence of calcium (Ca2+) ions in water.
Some of the rocks may contain gypsum (CaSO4.2H2O), anhydrite (CaSO4), Kieserite (MgSO4.H2O) or dolomite (CaCO3. MgCO3) which can dissolve to a limited extent in water. The presence of these dissolved substances also causes the water to be hard. These substances dissolve sparingly in water to form Ca2+ and Mg2+ ions which are responsible for water hardness as stated early.
Activity 1
Investigation of the causes of water hardness
Materials:
Test tube rack-
Five clean test tubes
Measuring cylinder (100cm3)
Calcium sulphate solution (1 mol dm-3)
Soap solution
Magnesium sulphate solution (1 mol dm-3)
Sodium sulphate solution (1 mol dm-3)
Potassium sulphate solution (1 mol dm-3)
Distilled water
Procedure:
Label five clean and dry test tubes as A, B, C, D and E. 2
Add 10 cm3 of 1.0M calcium, magnesium, sodium and potassium sulphate solutions and distilled water in each of the test tubes respectively.
Add 5 cm3 of soap in each test tube
Shake the test tubes well and place them in a test tube rack
Observe the amount of lather formed in each test tube, and if there is any precipitate (scum) formed.
Results:
Results of experiment showing minerals which cause water hardness
Test tube Salt present Ions present in solution of salt Lather or scum formed? Water hard or soft?
A calcium sulphate Ca2+, SO42- scum is formed Hard
B magnesium sulphate Mg2+ , SO42- scum is formed hard
C sodium sulphate Na+, SO42- lather is formed soft
D potassium sulphate K+, SO42- lather is formed soft
E distilled water no ions lather is formed soft
Interpretation of the results
From the result of experiment, we can conclude that scum is produced when either calcium or magnesium salt is present in water. So, high levels of calcium or magnesium ions in water are responsible for water hardness.
When the concentration of either of these minerals is over 150 milligrams per cubic decimeter (150 mg/dm3), water is considered to be hard. The upper limit allowed is 300 mg/dm3
Types of Hardness of Water
Types of Hardness of Water
Identify types of hardness of water
The hard water in some areas can be softened simply by boiling the water, but this is not true in all cases. This means that the hardness in water can be divided into two types – temporary and permanent hardness.
Temporary hardness
Temporary hardness in water is caused by dissolved calcium or magnesium hydrogencarbonates. The most important characteristic of temporarily hard water is that it can be softened by simply boiling. When the water is boiled, the soluble sodium hydrogencarbonate is decomposed to form the insoluble calcium carbonate.

The decomposition causes the “furring” of kettles, hot water pipes and shower heads. This means that the inside of kettles, pipes and shower heads become coated with a layer of calcium carbonate (limescale) caused by the decomposition of the hydrogencarbonate according to the equation above.
In many supermarkets, it is possible to buy a limescale remover. This is often a solution of methanoic acid (formic acid). This weak acid is strong enough to react with limescale but not with the metal. The insoluble limescale (carbonate) is probably dissolved to a soluble compound, calcium methanoate that can be flushed away with water.
2COOH(aq) + CaCO3(s)(insoluble)→ Ca(HCOO)2(aq) + H2O(l) + CO2(g)calcium methanoate (soluble)
Permanent hardness
Permanent hardness in water is caused by soluble sulphates and chlorides of calcium and magnesium (CaSO4, MgSO4, CaCl2 and MgCl2). This type of hardness cannot be removed by boiling the water. This is because boiling does not decompose the chlorides of calcium or magnesium. Such water may only be softened by chemical treatment or ion exchange methods
The Difference between Soft and Hard Water
Differentiate soft from hard water
Activity 2
Distinction between temporarily and permanently hard water
Materials:
Calcium carbonate
Dilute hydrochloric acid
4 test tubes
Test tube rack
1 litre of distilled water
Calcium chloride
Soap solution
Beaker
Heat source
Method:
Prepare carbon dioxide gas in the laboratory by mixing calcium carbonate with hydrochloric acid in a gas generator.
Bubble the gas through a suspension of calcium carbonate in water. Shake well as you bubble the gas until most of calcium carbonate has dissolved. Filter and divide the filtrate into two test tubes M and N.
Prepare a 0.5M solution of calcium chloride and divide it in two test tubes P and Q.
Prepare soap solution in a large beaker.
Arrange the four test tubes in a rack
Heat the solutions in test tubes M and P to boiling. Allow them to cool.7. Add 10cm3 of the soap solution to each of the four test tubes, M, N, P and Q. Shake well and allow to rest. Observe lathering and scum formation.
Note:
When calcium carbonate is reacted with dilute hydrochloric acid, carbon dioxide gas is produced.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
When carbon dioxide gas is bubbled through a suspension of calcium carbonate in water for a long time, the insoluble calcium carbonate dissolves to give the soluble bicarbonate, the presence of which makes the water hard.
CO2(g) + CaCO3(s) + H2O(l) → Ca(HCO3)2(aq)
The purpose of heating solutions in test tubes M and P was to try to remove water hardness. However, only the hardness in test tube M was merely removed by boiling because it contains the temporarily hard water.

The hardness in test tube P could not be removed by just boiling because it contained the hard water. Calcium chloride cannot be decomposed by heat. So, no change is expected after heating.
Results:
Scum was formed in test tubes N, P and Q but P and Q contained more scum than N.
Lather was formed in test tube M only.
Test tube M contained temporarily hard water and test tube P contained permanently hard water. The hardness in test tube M was removed by boiling while that in test tube P was not.
Test tubes P and Q contained permanently hard water. The hardness in this water could not be removed by mere boiling.
Treatment and Purification of Hard Water
Process of Hard Water Treatment and Purification
Examine process of hard water treatment and purification
Because of the problem it causes, hard water is often softened for use in factories, industries and homes. That means removing the dissolved calcium and magnesium ions. Described below are the methods of treating and purifying hard water.
Boiling
Boiling removes temporary hardness in water, as you saw early. Boiling causes calcium carbonate to precipitate. The hydrogencarbonate in water are decomposed to carbonates, which are insoluble in water

In this way, the calcium is removed, since the calcium carbonates being insoluble, takes no further part in the reaction. An insoluble calcium salt cannot cause hardness. However, this method uses a lot of fuel, which makes it expensive to do on a large scale.
Distillation
Distillation removes all impurities from water. This gets rid of both temporary and permanent hardness. In distillation, the water is boiled and the steam collected, cooled and condensed. Distilled water is pure and softest water. All the dissolved substances have been removed. Like boiling, it is an expensive option in terms of fuel used. But it is essential for some purposes, for example for laboratory experiments and for making drugs.
Addition of calcium hydroxide
Addition of calculated amounts of calcium hydroxide can remove temporary hardness. The quantity to be added should be properly calculated because excess would cause hardness on its own account. The amount of calcium hydroxide to be added is calculated based on knowledge of the hardness of water and the capacity of the reservoir (Clark’s method). The calcium hydroxide reacts with the hydrogencarbonates dissolved in water and precipitates as the insoluble calcium carbonates.
Ca(OH)2(s) slightly soluble + Ca(HCO3)2(aq) → 2CaCO3(s) + 2H2O(l)insoluble
Addition of sodium carbonate (washing soda)
Washing soda removes both temporary and permanent hardness by precipitating calcium carbonate. It reacts with calcium hydrogencarbonate (which causes temporary hardness) to form sodium hydrogencarbonate like this:
Na2CO3(aq)+ Ca(HCO3)2(aq)→ 2NaHCO3(aq))+ CaCO3(s)
It also reacts with calcium sulphate (which causes permanent hardness) to form sodium sulphate thus:Na2CO3(aq)+ CaSO4(aq)→ CaCO3(s)+ Na2SO4(aq)
These sodium salts are soluble, but do not cause the water to be hard. The calcium and magnesium ions are precipitated as insoluble calcium and magnesium carbonates. Ionically, the situation is like this:
Ca2+(aq)+ CO32-(aq)→ CaCO3(s)
Mg2+(aq)+ CO32-(aq)→ MgCO3(s)
Ion Exchange
Another method that removes both temporary and permanent hardness in water is the use of ion exchange resin. A typical ion exchanger is a container full of small beads. These beads are made of special plastic called ion exchange resin. The resin beads are porous and contain sodium ions. When hard water flows through the resin, the calcium and magnesium ions in the water are exchanged for the sodium ions and attach themselves to the resin. This process, therefore, removes calcium and magnesium ions from the water. They are replaced by sodium ions, which do not make the water hard.

An ion exchange column removes ca2+ and mg2+ ions from the water and replaces them with Na+ ions
When all sodium ions have been removed from the resin, it is regenerated by pouring a concentrated solution of sodium chloride through it. The sodium ions remove the calcium and/or magnesium ions off the resin and the ion exchanger is ready for the use again.Other ions could also be used instead of sodium for the resin. But sodium chloride is normally used to supply the sodium ions because salt is cheap.
Use of softeners
Many modern washing powders now have softeners added to them. The softeners are often phosphates. The phosphates ions react with calcium ions to form calcium phosphate and remove the hardness.3Ca2+(aq)+ 2PO43-(aq)→ Ca3(PO4)2(s)
The Importance of Hard Water Treatment and Purification
Describe the importance of hard water treatment and purification
The significance of water in daily life is well known to everyone. The water we obtain from natural sources is never pure. It contains dissolved minerals which render the water unfit for direct uses. The water from some sources contains calcium and magnesium compounds dissolved in it. These compounds are responsible for water hardness. To make the water fit for various uses, it is imperative to remove the hardness. The following points state why it is important to treat and purify hard water:
Hard water wastes soap. To get enough lather with hard water, it requires more soap than it does with soft water. So it is important to soften the water in order to save the soap and hence reduce the cost of washing. Laundry uses less soap and can be done at lower temperatures.
Treating and purifying hard water eliminates the possibility of forming limescale deposits in water boilers, kettles, washing machines, water heaters, shower heads and dish washers. The scale formed around the heating elements can cause the element to overheat and fail.
Treated and purified water leaves no scum on clothes during washing. Scum spoils the finishing of some fabrics. It forms nasty deposits (marks) on clothing that has been washed.
Softened water has the advantage of not blocking the water pipes. In industry, deposits of scales can block the pipes in boilers. This is a safety hazard as it could cause pressure to build up until there is an explosion. A similar coating can occur in hot water pipes at home and in central heating systems.
The Importance of Hard Water in Daily Life
State the importance of hard water in daily life
Hard water is not always disadvantageous. The following points explain the importance of hard water:
The dissolved calcium and magnesium salts improve the taste of water. Distilled water is tasteless and quite unpleasant to drink. This is why water-processing plants add some salts in the distilled water to make it tasteful.
Calcium dissolved in hard water is an essential mineral for growth of bones and teeth. It makes our teeth and bones hard, strong and resistant to shear and pressure.
In some places, old lead pipes are used for water supply. Lead is very poisonous, and a little of it can dissolve in soft water. But the carbonate (CO32-) or sulphate (SO42-) ions present in hard water reacts with lead to form a coating of lead carbonate or lead sulphate that prevents lead from dissolving. This prevents lead poisoning.
A coating of calcium carbonate inside pipes, boilers and radiators helps to prevent corrosion.
In recent years, it has been suggested that drinking hard water helps to prevent heart diseases. 6. It has also been found that hard water is good for brewing beer.
TOPIC 3: ACIDS, BASES, AND SALTS
Acids and Bases
The Natural Sources of Acids and Bases
Investigate the natural sources of acids and bases
In everyday life, we deal with many substances that chemists classify as acids. For example, orange juice and grapefruit juice contain citric acid. These juices, and others of the like, contain ascorbic acid, a substance more commonly known as vitamin C. Examples of natural sources of acids and the type of acids they contain are shown in table below.
Some natural sources acids
| Source | Type of acid present |
| Mineral acids (HCl, H2SO4, HNO3, etc.) | Minerals |
| Tobacco | Salicylic acid |
| Tea | Tannic acid |
| Coffee | Chlorogenic acid |
| Sugar beet | Glutaric and adipic acids |
| Blackberry | Isocitric acid |
| Spinach, tomato | Oxalic acid |
| Sour (fermented milk) | Lactic acid |
| Bee, ant and nettle stings | Methanoic acid (formic acid) |
| Grapes, bananas, tamarinds | Tartaric acid |
| Citrus fruits | Citric acid ( lemons and limes have particularly high concentrations of citric acid; it can constitute as much as 8% of the dry weight of these fruits) |
Acids have a sour taste. Vinegar, lemon juice, grapefruit juice and spoilt or fermented milk are all sour tasting because of the presence of acids. The acids present in animal and plant materials are known as organic acids.
Salads are often flavoured with vinegar, which contains dilute acetic acid. Boric acid is a substance that is sometimes used to wash the eyes.
In any chemistry laboratory, we find acids such as hydrochloric acid (HCl), sulphuric acid (H2SO4), and nitric acid (HNO3). These acids are called mineral acids because they can be prepared from naturally occurring compounds called minerals. Mineral acids are generally stronger and should be handled with great care, especially the concentrated acids, for they are very corrosive. They can eat away metals, skin and clothing. Nevertheless, some acids are not corrosive even when they are concentrated. They are called weak acids. Ethanoic acid is one example. It is found in vinegar. In general, organic acids are weaker than natural acids.
You can tell if a substance is acid or not by its effect on litmus. Litmus is a purple dye. It can be used as a solution, or on paper, called litmus paper.Litmus solution is purple. Litmus paper for testing acids is blue while that for testing bases is red in colour. Acids will turn litmus solution red. They will also turn blue litmus paper red.
Bases do not usually occur naturally. So they are not normally obtained from natural sources. However, they are prepared in the laboratory or in industry. Bases can be classified into oxides, hydroxides or carbonates. Therefore, bases can be defined as the oxides, hydroxides or carbonates of metals. Bases taste bitter. A bitter taste is a characteristic of all bases.
Most bases are insoluble in water. The bases which dissolve in water are known as alkalis. The most common alkalis are potassium hydroxide (KOH), sodium hydroxide (NaOH), calcium hydroxide, Ca(OH)2, and ammonium hydroxide (NH4OH), also known as ammonia solution.
Alkalis turn litmus solution blue and red litmus paper blue.A substance, such as litmus, which changes from one colour to another when mixed with an acid or base, is called an indicator. Table 3.2 shows how acids and bases (alkalis) affect the colours of different indicators. We can use this clue of colour changes to tell whether an unknown substance is an acid or base (alkali).
Some common indicator colour changes
| Indicator | Colour in acid | Colour in alkali (base) |
| Methyl orange | orange | yellow |
| Phenolphthalein | colourless | Pink |
| Litmus | red | blue |
| Bromothymol blue | yellow | blue |
The Reactions of Acids with Various Materials
Determine the reactions of acids with various materials
Acids react with different substances to produce different products. These reactions are best carried out by using dilute acid solutions. The following are some reactions of dilute acids with various substances.
Reaction with metals
Acids react with quite reactive metals (not very reactive ones) to produce salt and liberate a hydrogen gas.
metal + acid → salt + hydrogen
It is unsafe to try this reaction with veryreactive metals such as sodium or calcium. The reaction with such metals is so violent. Metals less reactive than lead, such as silver and gold have no reaction with dilute acids. Even with lead, it is difficult to see any reaction in a short time.
The salt produced when a dilute acid reacts with a metal depends on the acid and a metal used:
- Mg(s) + 2HNO3(aq) → Mg(NO3)2(aq) + H2(g)
- Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Reaction with carbonates
Acids react with carbonates to give salt, water and carbon dioxide. In general, all carbonates give off carbon dioxide when they react with acids.
acid + metal carbonate → salt + water + carbon dioxide
The normal methods of preparing carbon dioxide in the laboratory are based on this reaction. Dilute hydrochloric acid is reacted with marble chips (calcium carbonates):
2HCl(aq) + CaCO3(s) → CaCl2(aq) + H2O(l) + CO2(g)
Reaction with oxides and hydroxides (alkalis)
Hydroxides: acids react with alkalis, forming salt and water:
NaOH(aq) + HNO3(aq) → CaCl(aq) + H2O(l)
Oxides: They also react with metals oxides, forming salt and water:
ZnO(s) + 2HCl(aq) → ZnCl2(aq) + H2O(l)
The bases (oxides, hydroxides) all react in the same way with acids, and in the process, salts are formed. This type of reaction is known as neutralization reaction. It can be summarized up in a general equation:
acid + base → salt + water
Reaction with hydrogencarbonates (bicarbonates)
Acids react with hydrogencarbonates, forming salt, water and liberating carbon dioxide gas:
NaHCO3(aq) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g)
The Reactions of Alkalis with Various Materials
Determine the reactions of alkalis with various materials
Alkalis react with acids to produce salt and water. All alkalis, except ammonia solution, will react with ammonium compounds liberating ammonia gas. Aqueous solutions of alkalis will precipitate the insoluble hydroxides of other metals from the solutions of metal salts. Caustic alkalis attack aluminium, zinc and lead to form salts. They react with carbon dioxide to form carbonates.
The Reactions of Bases with Various Substances
Determine the reactions of bases with various substances
Characteristic reactions of bases
Dissolution in water
Most bases are insoluble in water. Some are soluble in water. Soluble bases are known as alkalis. The commonest alkalis are sodium hydroxide, (NaOH), calcium hydroxide, Ca(OH)2, potassium hydroxide, (KOH), and ammonium hydroxide or ammonia solution, (NH4OH). All alkaline solutions contain hydroxyl ions, OH-. In sodium hydroxide solution, the ions are produced like this:
NaOH(aq)→ Na+(aq)+ OH-(aq)
Like acids, alkalis can also be classified as strong or weak. Ammonia solution is a weak base because it ionizes just partially:
The rest of the bases are strong bases because they ionize fully into ions in solution.
Reaction with acids
Bases react with acids to produce salt and water. Refer to the reactions of acids with oxides and hydroxides discussed early.
Reaction with ammonium compounds
Alkalis turn litmus solution blue and red litmus paper blue.A substance, such as litmus, which changes from one colour to another when mixed with an acid or base, is called an indicator. Table 3.2 shows how acids and bases (alkalis) affect the colours of different indicators. We can use this clue of colour changes to tell whether an unknown substance is an acid or base (alkali).
Reaction with aqueous salts of metals
Aqueous solutions of alkalis will precipitate the insoluble hydroxides of other metals from the solutions of metal salts. Only NH4OH, KOH and NaOH are soluble enough in water.
All other hydroxides are insoluble and can be precipitated from aqueous solution by these three alkalis.
- When sodium hydroxide solution is added to copper (II) sulphate solution, a pale blue precipitate of copper (II) hydroxide is formed. CuSO4(aq) + 2NaOH(aq) → Cu(OH)2(s) + Na2SO4(aq)
- Another example is the reaction between potassium hydroxide and iron (II) chloride, which precipitates iron (II) hydroxide. FeCl3(aq) + 3KOH(aq) → Fe(OH)3(s)+ 3KCl(aq)
Reaction with metals
Caustic alkalis attack very few metals. The metals known to be attacked by the alkalis are aluminum, zinc and lead, where the aluminate, zincate and plumbate (II) are formed respectively. The aluminum will react thus:2Al(s) + 6NaOH(aq) + 6H2O(l) → 2Na3Al(OH)6(aq) + 3H2(g)(sodium aluminate)
Reaction with carbon dioxide
When carbon dioxide gas is bubbled through aqueous solutions of the caustic alkalis, the carbonates are formed.
2NaOH(aq) +CO2(g) → Na2CO3(aq) + H2O(l).With excess of the gas, the hydrogencarbonates are formed.Na2CO3(aq)+ H2O(l) + CO2(g) → 2NaHCO3(aq)
Reaction with chlorine
Chlorine reacts with excess of cold dilute caustic alkalis to form the hypochlorite, (NaClO or KClO).2NaOH(aq) + Cl2(g) → NaCl(aq)+ NaClO(aq) + H2O(l).2KOH(aq) + Cl2(g) → KCl(aq) + KClO(aq) + H2O(l)
If excess chlorine is bubbled through hot concentrated solutions of caustic alkalis, the chlorates are formed, (NaClO3 or KClO3).
6NaOH(aq) + 3Cl2(g) → 5NaCl(aq) + NaClO3(aq)+ 3H2O(l).6KOH(aq) + 3Cl2(g) → 5KCl(aq) + KClO3(aq) + 3H2O(l)
Applications of acid-base neutralization in everyday life
Applications of acid-base neutralization in everyday life
Acid-base neutralization has many applications in everyday life. The following are some of these applications:
Indigestion and pain relief
The dilute hydrochloric acid produced in your stomach is used for digestion and killing bacteria that might have been swallowed together with food or taken with water. However, excess acid causes indigestion, which can be painful. To ease the pain, we take an anti-acid treatment. Anti-acids are a broad group of compounds with no toxic effects on the body. They are used to neutralize the effects of acid indigestion.
Some of these anti-acids such as milk of magnesia [insoluble magnesium hydroxide, Mg(OH)2] help to neutralize and hence counteract the excess acid in the stomach. This treatment, therefore, prevents indigestion and pains. The neutralization reaction equation is:
Mg(OH)2(aq) + 2HCl(aq)→ MgCl2(aq) + 2H2O(l)
Other anti-acids such as “Alka-Seltzer” contain soluble materials, including sodium hydrogencarbonate. These tablets also contain some citric acid (a solid acid). On adding water, the acid and some of the sodium hydrogencarbonate react, producing carbon dioxide gas. This helps to spread and dissolve the other less soluble material. When taken, more sodium hydrogencarbonate neutralizes the excess hydrochloric acid in the stomach, thus easing digestion.
Some anti-acid tablets also contain painkiller to relieve pain. “Soluble aspirin” tablets dissolve and work in a similar way to “Alker-Seltzer” tablets. Vitamin C (ascorbic acid) can be added to the tablets. Note that it is important to add water to start the action of the acid.
Descaling kettles
The limescale (CaCO3) is formed inside boilers, kettles and water heaters when hard water is boiled. The limescale can be removed by treatment with an acid that is strong enough to react with CaCO3, but not strong enough to damage the metal. Vinegar can be used to discale kettles. Commercial “discalers” use other acid solutions such as methanoic acid
Prevention of tooth decay
Food remnants sticking onto teeth (plaque), after eating especially sugary food is acted upon by bacteria in your mouth. The pH of a sugar solution is 7. However, bacteria in your mouth break down the sugar in plaque to form acids, for example lactic acid. These acids lower the pH. Tooth decay begins when the pH falls below 5.8. The acid attacks the tooth enamel.
To help prevent tooth decay many types of toothpaste contain basic substances to neutralize the acids produced by these bacteria in your mouth. The pH of these basic substances is alkaline (higher than 7). The pH of saliva is slightly alkaline (pH 7.4), so it can also help to counteract the acid, particularly after a meal. After eating a sweat, for example, it takes about 15 minutes for saliva to raise the pH above 5.8, and stop further decay.
Soil treatment
Most plants grow best when pH of the soil is close to 7. They prefer the pH of between 6.5 and 7.0. If the soil pH is below 6.0, the soil is too acidic. Above the pH of 8.0, the soil is too alkaline. If the soil is too acidic or too alkaline, the plants grow poorly or not at all.
Chemicals can be added to the soil to adjust its pH. Most often, if the soil is too acidic, it is usually treated by liming. In this context, liming means addition of quicklime (calcium oxide), slaked lime (calcium hydroxide) or powdered chalk or limestone (calcium carbonate) to an acidic soil. These compounds (bases) have the effect of neutralizing the acidity of the soil.
If the soil is too alkaline, acids such as sulphuric acid, nitric acid or hydrochloric acid may be added to the soil to neutralize excessive alkalinity. However, these compounds are very expensive and hence uneconomical to apply on large-scale basis.
Insect stings treatment
When a bee stings someone, it injects an acid liquid into the skin. The bee sting, which is acidic in nature, can be neutralized by rubbing on calamine solution, which contains zinc carbonate or baking soda, which is sodium hydrogencarbonate. These compounds are basic in nature and so have the effect of neutralizing the acid in the sting.
Wasp stings are alkaline in nature, and can be neutralized with vinegar, which contains ethanoic acid. Ant and nettle stings contain methanoic acid. These may be neutralized by rubbing an extract squeezed from crushed onion leaves (which contain basic compounds) on the affected skin. The acid in the sting can also be neutralized by applying weak alkalis such as ammonia solution, ash extract, baking powder, etc.
Factory wastes treatment
Liquid wastes from factories often contain acid. If it reaches a river, lake or ocean, the acid will kill fish and other aquatic life. This can be prevented by adding slaked lime (calcium hydroxide) to the waste, to neutralize the acid before being dumped into water bodies.
Indicators
An Indicator from Locally Available Materials
Explain an indicator from locally available materials
Certain coloured substances (many extracted from plants) have been found to change colour if added to an acid or alkaline solution. The colour change is reversed if the acid or alkali is neutralized. Substances that behave like this are known as indicators.
Coloured extracts can be made from red cabbage or blackberries, but probably the most used indicator is litmus. This is extracted from lichens.
Litmus is purple in a neutral solution. When added to an acid solution, it turns red. Changing this red colour of litmus needs a chemical reaction. The molecules of the indicator are usually changed in the presence of the acid. Substances with the opposite chemical effect to acids are needed to reverse the change, and these are called alkalis. They turn litmus solution to blue. Litmus can also be used in paper form, in which case it is called litmus paper. Here it comes in the blue and red forms. Litmus is a single chemical compound. It gives a single colour change.
Litmus is not the only single indicator that chemists find useful. Others that are used frequently are phenolphthalein and methyl orange. These indicators give different colour changes when in acidic and alkaline solutions (see table 3.2).Another commonly used indicator is the universal indicator (or full-range indicator). This is made from a mixture of dyes. Such an indicator is useful because it gives a range of colours (“spectrum”) depending on the strength of the acid or alkali added (see table 3.3)
With a universal indicator, different acids produce a range of different colours. Indeed, solutions of the same acid with different concentrations (pH) give different colours.
The more acidic solutions (for example battery acid) turn the universal indicator bright red. A less acidic solution (for example vinegar) will only turn it orange-yellow. There are also colour differences produced with different alkali solutions. The most alkaline solutions give a violet colour while the less alkaline solutions give a blue colour.
We learned that many indicators are extracted from plants. Flowers and leaves of different plants have different colours. These plant organs may be used to prepare indicators locally.
Activity 1
To prepare indicators from local plant materials
Procedure:
- Collect flowers from different plants in your local area. You may use coloured leaves if the coloured flowers are not available.
- Crush the flowers/leaves in a motor and pestle to make a fine paste.
- Add ethanol to the paste to wash out chlorophyll. Add about 10cm3 of ethanol per gram of pestle used.
- Grind the mixture to a very fine paste so that the ethanol can penetrate the broken plant cells fully.
- Place the mixture in the sun or heat gently to evaporate off ethanol. Make sure most of the ethanol has evaporated.
- Filter the mixture to obtain a clear but coloured filtrate. To obtain as much extract as possible, squeeze the paste in a clean piece of cloth and collect the juice in a beaker. The liquid you obtain is your indicator.
- Arrange test tubes in a rack and label them A, B, C D and E.
- Pour sodium hydroxide, dilute hydrochloric acid, limewater, lemon juice, vinegar and washing soda in test tubes A, B, C, D and E respectively.
- Add two to three drops of the prepared indicator in each of the test tubes. Observe and record the colour changes.
Questions from the experiment
- What was the colour of your indicator?
- Write down the colour changes in each of the test tubes A to E.
- Which substance showed a sharp colour change?
- Perform a similar experiment using a ready-made universal indicator and observe whether there is any difference in colour changes between this commercial indicator and that one prepared from local plants.
The Acidity and Alkalinity of Substance Using Indicators
Test the acidity and alkalinity of substance using indicators
The Strengths of Acids and Bases
There is a big difference between the strength of an acid or base and its concentration. An acid or alkaline solution is said to be concentrated if it contains a large amount of it in a small amount of water. A dilute acid or base (alkali) has a small amount of it in a lot of water. The concentration of an acid or base tells us how much of it is dissolved in a certain volume of solution. The concentration is normally expressed in grams per litre (g dm-3) or moles per litre (mol dm-3).
The strength of an acid or alkali expresses its dissociation in water. Strong acids or alkalis will dissociate completely in water to form ions. Examples of strong acids are sulphuric acid, hydrochloric acid, nitric acid and phosphoric acid. Weak acids include ethanoic acid, carbonic acid and methanoic acid. Examples of strong alkalis include potassium hydroxide, sodium hydroxide, calcium hydroxide and ammonium hydroxide. Weak bases include ammonia solution and sodium hydrogencarbonate.
A strong acid or alkali forms many ions in water. The number of hydrogen ions, H+, formed when it dissociates in water, determines the strength of an acid. The strength of an alkali depends on the number of hydroxyl ions, OH-, formed when it dissociates in water. Strong acids and alkalis will form many H+ and OH- ions respectively. Weak acids or bases will form very few of the respective ions.
Likewise, the term weak acid or base should not be confused with the term dilute acid or base. A weak acid dissociates in water only very slightly to form very few protons, H+. A weak alkali also dissociates very slightly to form very few hydroxyl ions, OH-.
The Concept of an Indicator
Describe the concept of an indicator
You have seen that single indicators change their colours only once when put in different acid and alkaline solutions. The single indicators most commonly used include litmus, phenolphthalein and methyl orange.
On the other hand, universal indicators show a range of colour changes depending on the strength of an acid or base.
Single indicators can only tell us whether a certain solution is an acid or an alkali. These types of indicators cannot be used to compare two acids or two alkalis with different strengths. Litmus paper, for example, cannot be used to compare the strengths of sulphuric acid and ethanoic acid. Both acids will change the blue litmus paper to red. Likewise, you cannot compare the strengths of aqueous ammonia solution (NH4OH) and sodium hydroxide by just using a litmus paper. They will both turn to redlitmus paper to blue.
A universal indicator can be used to measure strengths of different acids and alkalis. This indicator is a mixture of simple indicators. Instead of changing colour just once, it changes colour a number of times depending on the degree of acidity or alkalinity of the substances tested.
The pH scale is a convenient means of expressing the acidity and alkalinity in liquids. The pH scale is a numerical scale used to indicate the relative strengths of acidic or basic solutions in terms of relative amount of hydrogen ions (protons) or hydroxyl ions in solutions. The scale ranges from 0 to 14.
Acidic solutions will have pH values less than 7.0 and alkaline solutions will have pH values greater then 7.0. All neutral liquids e.g. pure water have pH of 7.0. Table 3.3 shows the pH and strengths of acidic and alkaline solutions and the associated indicator colour changes.
Colours of the universal indicator in different acidic and alkaline solutions
| pH range | Colour | Strength |
| 1, 2, 3 | Red | Strongly acidic |
| 4 | Orange | |
| 5, 6 | Yellow | Weakly acidic |
| 7 | Green | Neutral |
| 8, 9 | Blue Indigo | Weakly alkaline |
| 10, 11, 12, 13, 14 | Purple/violet | Strongly alkaline |
Remember that there is no clear dividing line between the pH ranges as apparently shown in the above table. This means that you may have substances with, for example, pH 1.2, 1.5, 3.5, 4.4, 5.6, 8.4, etc. The table just tries to simplify the concept of acidity and alkalinity of acid and alkaline solutions.
SALT
The Natural Source of Salts in Daily Life
Investigate the natural source of salts in daily life
A salt is a substance formed when some or all of the hydrogen atoms of an acid are replaced by a metal or ammonium ion. A salt, therefore, may be defined as a compound in which the replaceable hydrogen of an acid has been wholly or partially replaced by a metal.
In sodium chloride (NaCl), for example, the hydrogen atom of hydrochloric acid (HCl) has been wholly replaced by an atom of sodium. In magnesium sulphate (MgSO4) and sodium sulphate (Na2SO4), both hydrogen atoms of sulphuric acid (H2SO4) have been replaced by one atom of magnesium and two atoms of sodium respectively. In sodium hydrogen sulphate (NaHSO4), only one out of two hydrogen atoms has been replaced by an atom of sodium. This type of a salt is called an acid salt, because it still contains a replaceable hydrogen atom.
Many chemical compounds may be classified as salts. The salt most familiar to every body is table salt (sodium chloride). Baking soda is the salt, sodium bicarbonate (NaHCO3). Magnesium sulphate (also called Epsom salt) is often found in the home.
In general, salts are ionic impounds that are composed of metal and non metal ions. For example, sodium chloride is is composed of metallic sodium ions (Na+) and non-metallic chloride ions (Cl-). Some salts are made of metallic and non-metallic radicals e.g ammonium nitrate (NH4NO3) is composed of ammonium radical (NH4+) and nitrate radical (NO3-).
There is a wide range of types and natural sources of salts. Common salt is mined from underground deposits. The salt obtained from such a source contains sodium chloride mixed with rock impurities.
The other source of sodium chloride is seawater. The salty taste of seawater is due to the presence of salts such as sodium chloride and magnesium bromide. However, there are many different types of salts present in seawater, though in small proportions, as shown in the table below (table 3.5)
| Salt | Formula | Percentage composition |
| Sodium chloride | NaCl | 2.72 |
| Magnesium chloride | MgCl2 | 0.38 |
| Magnesium sulphate | MgSO4 | 0.17 |
| Calcium sulphate | CaSO4 | 0.13 |
| Potassium chloride | KCl | 0.09 |
| Calcium chloride | CaCO3 | 0.01 |
| Magnesium bromide | MgBr2 | 0.01 |
Sodium nitrate (Chile saltpetre), NaNO3 and calcium carbonate, CaCO3 are found in underground deposits. Calcium carbonate occurs naturally as marble, limestone or chalk in the ground from which it can be mined mechanically. What other natural sources of salts do you know?
Types of Salts
Salts may be classified according to their mode of formation. The following are types of salts grouped according to their mode of formation:
Normal salt:- This is a salt formed when all of the replaceable hydrogen atoms of an acid have been replaced by a metal atom e.g. sodium chloride is a normal salt because all hydrogen atoms are replaced from an acid during its formation.
2Na(s) + 2HCl(aq)→ 2NaCl(aq) + H2(g)
Other normal salts include magnesium chloride (MgCl2), potassium chloride (KCl), copper (II) sulphate (CuSO4), sodium sulphate (Na2SO4), sodium carbonate (Na2CO3), trisodium phosphate (Na3PO4), etc.
Acid salt:- An acid salt is a salt formed when part of the replaceable hydrogen atoms of an acid are displaced by a metal e.g., sodium bisulphate (NaHSO4) is an acid salt.
H2SO4(aq) + NaOH(aq)→ NaHSO4(aq) + H2O(l)
Other examples of acid salts include sodium hydrogensulphate, (NaHSO4), sodium hydrogensulphide, (NaHS) and sodium hydrogencarbonate (NaHCO3). Since acid salts contain hydrogen ions, they exhibit some acidic properties. Hence, they behave like acids, for example:
- they react with bases to form salts and water only. NaHSO4(aq) + NaOH(aq)→ Na2SO4(aq) + H2O(l)
- they react with carbonates to yield carbon dioxide.2NaHSO4(aq) + Na2CO3(aq)→ 2Na2SO4(aq) + H2O(l) + CO2(g)
Basic salt:- A basic salt is formed by the action of an acid with higher proportions of the base, than is necessary for the formation of a normal salt.
Examples of basic salts are:
- Basic copper carbonate, CuCO3.Cu(OH)2
- Basic lead carbonate (white lead), PbCO3.Pb(OH)2
- Basic magnesium chloride, MgCl2.Mg(OH)2
- Basic zinc chloride, ZnCl2.Zn(OH)2
A basic salt may also be formed by the partial replacement of the hydroxyl groups of a diacidic or triacidic base by an acid radical.
Pb(OH)2(s) [lead hydroxide] + HNO3(aq) [nitric acid] → Pb(OH)NO3(s) [basic lead nitrate]+ H2O(l) [water]
Basic salts are usually insoluble in water. Such salts are formed by the close association of two simple salts, when crystallized from a solution of a mixture of the two.
The Solubility of Different Salts in the Laboratory
Analyse the solubility of different salts in the laboratory
Some salts are more soluble in water than others are. However, other salts are insoluble in water. The knowledge of solubility of different salts in water is very important because it can help us prepare different salts in the laboratory by such methods as precipitation, direct combination (synthesis), crystallization and so forth.
As regards to solubilities, salts can be classified into two groups: salts which are soluble in water (soluble salts) and salts which do not dissolve in water (insoluble salts). Table 3.6 summarizes the solubility of different salts in water.
The patterns of solubility for various types of salts
| Soluble salts | Insoluble salts |
| 1. All sodium, potassium and ammonium salts. | silver, mercury(I) and lead chlorides barium, lead (II) and calcium sulphates but other common carbonates are insoluble.but other common hydroxides are insoluble. |
| 2. All nitrates of metals | |
| 3. All chlorides except .……………........ | |
| 4. All sulphates except………………….. | |
| 5. Sodium, potassium, and ammonium carbonates…………………………... hydroxides………………………….…… | |
| 6. Sodium, potassium and ammonium |
Salts in the Laboratory
Prepare salts in the laboratory
Several methods are available for the preparation of salts. The solubilities of the prepared salts determine their methods of preparation. Hence, in the choice of a method of preparation of a particular salt, one has to be acquainted with its solubility properties.
Soluble salts are usually prepared by methods which involve crystallization. In this method, as the name suggests, resultant salts are in the form of crystals.
Insoluble salts are usually prepared by methods which involve precipitation. These methods are sometimes referred to as double decomposition. To precipitate an insoluble salt, you must mix a solution that contains its positive ions with the one that contains its negative ions.
Salts may also be prepared by direct combination (or synthesis). For example, magnesium chloride may be prepared in the laboratory by heating magnesium in a stream of chlorine.
Mg(s) + Cl2(g) → MgCl2(s)
Preparation of soluble salts
Soluble salts may be prepared by any of the following methods:
- Reaction between an acid and an alkali:In this method, a dilute acid is added to an alkali in the appropriate volume ratio. The reaction between an acid and an alkali is termed as neutralization. For example, sodium chloride may be prepared by the following neutralization reaction:NaOH(aq) + HCl(aq)→ NaCl(aq) + H2O(l). Both reactants are soluble, and no gas is given off during the reaction. So, it is difficult to know when the reaction is over. In this case, you have to use an indicator. A universal indicator or litmus could be used, but even better is phenolphthalein. This is pink in alkaline solution, but colourless in neutral or acidic solutions.
- Reaction of a metal with an acid:This is another general method for preparing salts. For example, zinc sulphate can be made by reacting dilute sulphuric acid with zinc:Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g).However, this method is not suitable for all metals or all acids. It is good for preparing salts of fairly reactive metals such as magnesium, aluminium, zinc and iron. However, the reactions of highly reactive metals like sodium, potassium and calcium with acids are very violent and dangerous. The reaction with lead is too slow. Copper, silver and gold do not react at all.
- Reaction of a metal oxide with an acid.Metal oxides, as you studied early, react with dilute acids to produce salts. Copper oxide is an insoluble base. Although copper will not react with dilute sulphuric acid, copper (II) oxide will. The salt that forms is copper (II) sulphate.CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l)
- Reaction of a metal carbonate with an acid.The reaction between metal carbonates and dilute acids are accompanied with evolution of carbon dioxide gas. The evolution of a gas can be used to indicate when the reaction is over. An example of such reactions is the reaction between calcium carbonate and dilute hydrochloric acid. CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l)+ CO2(g)
General methods of preparing soluble salts
The above methods for preparing soluble salts are specific for each method mentioned. Generally, soluble salts may be prepared by two broad methods.
Method 1:This route is essentially the same whether starting with a solid metal, a solid base (oxide) or a solid carbonate. The route can be divided into four stages:
- Stage 1:An excess (more than enough) of the solid is added to the acid and allowed to react. Using an excess of the solid makes sure that all the acid used up. If it is not used up at this stage, the acid would become more concentrated when the water is evaporated later (stage 3).
- Stage 2:The excess solid is filtered out after the reaction is completed.
- Stage 3: The filtrate is gently evaporated to concentrate the solution. This can be done on a heated water bath. Do not heat so strongly or “spitting” might take place.
- Stage 4:The concentrated solution is cooled down to let the crystals form. Filter off the crystals. Wash them with a little distilled water. Dry the crystals carefully between the filter papers.
Method 2:This method (titration method) involves the neutralization of an acid with an alkali (for example sodium hydroxide) or a soluble carbonate (for example sodium carbonate). Since both the reactants and the products are colourless, an indicator is used to find the neutralization point or end point(when all the acid has just been neutralized). Once the end point is reached, the resulting salt solution is evaporated and cooled to form crystals as described in method 1.
General methods for preparing insoluble salts
Some salts are insoluble in water (for example silver chloride and barium sulphate – see table 3.6). Such salts are generally prepared by ionic precipitation.Precipitation is the sudden formation of a solid either: when two solutions are mixed; or when a gas is bubbled into a solution.
For example, barium sulphate can be prepared by adding a solution of a soluble sulphate (for example sodium sulphate) to a solution of a soluble barium salt (for example barium chloride). The insoluble barium sulphate is formed immediately. This solid falls to the bottom of the container as a precipitate (figure 3.2). The precipitate can be filtered off. It is then washed with distilled water and dried in a warm oven. The equation for the reaction is:
BaCl2(aq) + Na2SO4(aq)→ BaSO4(s) + 2NaCl(aq)
This shows how important the state symbols can be - it is only through state symbols that we can tell this equation shows a precipitation.
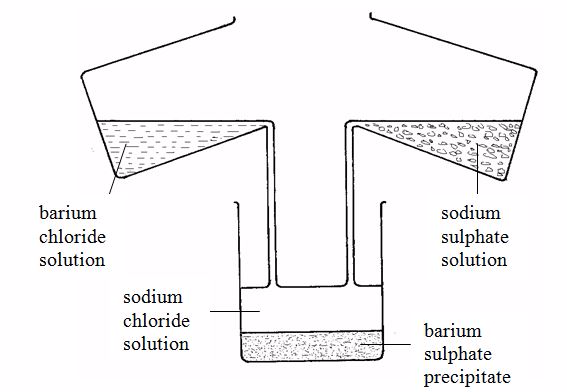
Barium sulphate could also be made from barium nitrate and sodium sulphate, for example, since these salts are both soluble. As long as barium and sulphate ions are present, barium sulphate will be precipitated.
Ba2+(aq) + SO42-(aq) →BaSO4(s)
Precipitation reactions are often used in a qualitative analysis to identify salts such as chlorides, iodides and sulphates.
Preparation of salts by direct combination (synthesis)
Some soluble and insoluble salts can be made directly by reacting two elements together. This is called combination (or synthesis). This type of reaction is mainly possible for metal chlorides, bromides and iodides. For instance, if a piece of burning sodium is lowered into a gas jar of chlorine, the two react violently to produce a white powder of sodium chloride:
2Na(s) + Cl2(g) → 2NaCl(s)
Other chlorides can also be prepared by combination, for example, iron (III) chloride and aluminium chloride can be made by heating iron and aluminium metals in stream of chlorine:
2Fe(s) + 3Cl2(g) → 2FeCl3(s)
2Al(s) + 3Cl2(g) → 2AlCl3(s)
The reaction between ammonia gas and hydrogen chloride gas to produce ammonium chloride is also a synthesis reaction.
NH3(g) + HCl(g) → NH4Cl(s)
Direct combination reactions do not produce crystals of the salt, but only a powder.
The Effects of Heat on Salts
Examine the effects of heat on salts
When different salts are heated, they behave in different manners. The crystals of some salts contain water of crystallization. When these hydrated salts are heated, their water of crystallization is driven off as steam. The crystals then lose their shape and become a powder. The following are few examples of hydrated salts:
| Salt formula | Chemical name |
| CuSO4.5H2O | Copper (II) sulphate five water |
| Na2CO3.I0H2O | Sodium carbonate ten water |
| MgCl2.6H2O | Magnesium chloride six water |
| FeCl3.6H2O | Iron (III) chloride six water |
| FeSO4.7H2O | Iron (II) sulphate seven water |
| CoCl2.6H2O | Cobalt (II) chloride six water |
| MgSO4.7H2O | Magnesium sulphate seven water |
| CaSO4.2H2O | Calcium sulphate two water |
Sulphates
Sulphates of potassium, sodium, calcium, lithium and magnesium are stable to heat and do not decompose when heated. Other sulphates decompose to give the oxide and sulphur trioxide gas except iron (III) sulphate which decomposes to give sulphur dioxide and sulphur trioxide.
Copper (II) sulphate five water crystals are blue in colour, but when heated, they are dehydrated to form a white powder:
CuSO4.5H2O(s)hydrated (blue)→ CuSO4(s)anhydrous (white) + 5H2O(g)
Crystals that have lost their water of crystallization are called anhydrous. If water is added back to the anhydrous copper (II) sulphate powder, the powder turns into blue crystals again and heat is evolved. This can be used as a qualitative test for water.If the white, anhydrous powder is further heated strongly, it decomposes to black copper (II) oxide:
CuSO4(s)white→ CuO(s)black+ SO3(g)
Hydrated iron (II) sulphate is green in colour. When heated, it loses all its water of crystallization and changes colour from green to white:
FeSO4.7H2O(s)green→ FeSO4(s)white + 7H2O(g)
When heated even more strongly, the white powder decomposes to form a black oxide:
2FeSO4(s)white→ Fe2O3(s)black+ SO2(g) + SO3(g)
Iron (III) sulphate decomposes on heating to form slightly different products:
Fe2(SO4)3(s) → Fe2O3 + 3SO3(g)
Chlorides
The chlorides of most metals are hydrated except those of potassium, lead, mercury and silver. Hydrated chlorides do not usually give the anhydrous salt when heated. Instead, a chemical change termed as hydrolysis normally occurs. The reaction is accompanied by the evolution of steam and hydrogen chloride gas, and the formation of the basic chloride or oxide. When, for example, hydrated magnesium chloride is heated, its basic chloride is formed:
MgCl2.6H2O(s) → Mg (OH)Cl(s) + HCl(g) + 5H2O(g)
The same case applies when hydrated calcium chloride is heated. However, when hydrated aluminum chloride is heated, it does not produce the anhydrous salt. Instead, the oxide is formed thus:
2AlCl3.6H2O(s) → Al2O3(s) + 6HCl(g) + 3H2O(g)
Ammonium chloride sublimes when heated. The reaction is reversible and the products may recombine on cooling to form the salt back.
NH4Cl=⇔NH3(g) + HCl(g)
Carbonates and hydrogencarbonates
The carbonates of potassium and sodium are very stable to heat. They do not decompose even when heated to very high temperatures. All other carbonates decompose when heated to give the oxide and carbon dioxide:
CaCO3(s) ⇔CaO(s) + CO2(g)
CuCO3(s) → CuO(s) + CO2(g)
However, there are very few and exceptional carbonates that do not behave like this. Ammonium carbonate, for example, decomposes readily when heated to give ammonia gas, water vapour and carbon dioxide gas:
(NH4)2CO3(s) → 2NH3(g) + H2O(g) + CO2(g)
All hydrogencarbonates decompose on heating to give the carbonates, water vapour and carbon dioxide:
2NaHCO3(s) → Na2CO3(s) + H2O(g) CO2(g)
Nitrates
When heated, potassium and sodium nitrates decompose to give the nitrite and oxygen:
2KNO3(s) → 2KNO2(s) + O2(g)
2NaNO3(s) → 2NaNO2(s) + O2(g)
The nitrates of common heavy metals (such as Pb, Al, Ca, Mg, Zn and Cu) decompose on heating to give the oxide, nitrogen dioxide and oxygen:
2Pb(NO3)2(s) → 2PbO(s) + 4NO2 (g) + O2 (g)
2Ca(NO3)2(s) → 2CaO(s) + 4NO2 (g) + O2 (g)
The nitrates of silver and mercury are completely decomposed to the metal, nitrogen dioxide and oxygen:
2AgNO3(s) → 2Ag (g) + 2NO2 (g) + O2 (g)
Hg(NO3)2(s) → Hg(l) + 2NO2(g) + O2(g)
Ammonium nitrate is decomposed by heat into dinitrogen oxide and water:
NH4NO3(s) → N2O (g) + 2H2O (l)
Hydroxides
Potassium and sodium hydroxides are very stable to heat. They do not decompose even when heated strongly. All other hydroxides decompose to give the oxide and water vapour, e.g.:
Ca(OH)2(s) → CaO(s) + H2O(g)
DELIQUESCENCE, EFFLORESCENCE AND HYGROSCOPY
Deliquescence
Deliquescence is the absorbing of moisture from the atmosphere by a solid to form a solution.If calcium chloride (CaCl2) is exposed to air, it absorbs water vapour from the atmosphere and eventually dissolves. Its tendency to absorb water vapour explains why it is used as a drying agent for gases (not ammonia, because it combines with the gas).
Solid sodium hydroxide is also deliquescent. On exposure to air, pellets of sodium hydroxide quickly become shiny and then sticky as they absorb water vapour from the atmosphere. Eventually the sodium hydroxide pellets absorb moisture from the atmosphere so much that they dissolve to form a solution of sodium hydroxide.
Copper (II) nitrate and zinc chloride are the other deliquescent salts. Pure table salt (NaCl) is not deliquescent. However, if the salt is directly obtained from the sea, it is deliquescent. The salt from the sea contains magnesium chloride as one as the impurities. It is this magnesium chloride salt that deliquesces and not sodium chloride.
Hygroscopy
Some substances tend to absorb water vapour from the air but do not change their physical states. Copper (II) oxide and calcium oxide are both hygroscopic solids because they can absorb moisture from the atmosphere and yet retain their solid states. Because of this behaviour, calcium oxide is used as a drying agent, which absorbs moisture from gases prepared in the laboratory.
Concentrated sulphuric acid is a hygroscopic liquid. When exposed to air, the acid absorbs water vapour from the atmosphere diluting itself to absorb 3 times its original volume.
Therefore, hygroscopy may be defined as the tendency of a substance to absorb water vapour from the atmosphere without changing its physical states.The word hygroscopy is a general term applied to all substances that absorb water vapour from the air. Any substance that can take up moisture from the atmosphere is said to be hygroscopic in nature.
Efflorescence
Efflorescence is the tendency of a hydrated substance to lose the water of crystallization to the atmosphere. Some salt crystals give out some or all of their water of crystallization to the atmosphere when exposed to air. Such substances are said to be efflorescent and the process of water loss is known as efflorescence. Sodium carbonate ten water (washing soda) is a good example of an efflorescent substance. If washing soda crystals are exposed to open air at room temperature, they lose some of the water of crystallization. The solid loses nine of its ten molecules of water of crystallization to the air. One molecule of water, which remains fixed, can be removed only by strong heating.
Na2CO3.10H2O(s)crystals → Na2CO3.H2O(s)powder + 9H2O(g)
The crystal lattice is broken down as the salt loses its after of crystallization. Thus, transparent crystals of hydrated sodium carbonates become white and powdery on the surface.
Another efflorescent compound is Glauber’s salt, sodium sulphate ten water (Na2SO4.10H2O). On exposure to air, it loses the whole of its water of crystallization to the air.
Na2SO4.10H2O(s) → Na2SO4(s) + 10H2O(g)
Iron (II) sulphate seven water (FeSO4.7H2O) is also efflorescent.
The Uses of Different Types of Salts in Everyday Life
Explain the uses of different types of salts in everyday life
There is a wide range of salts. A great number of them play an important role in our everyday life. The following are the uses of some salts:
Sodium chloride (common salt)
Sodium chloride often called common salt or table salt, is essential for life and is an important raw material for industries. At home, it is used for cooking, that is, flavouring different foods.Biologically, it has a number of functions: it is involved in muscle contraction; it enables the conduction of nerve impulses in the nervous system; it regulates osmosis (the passage of solvent molecules through membranes); and it is converted into the hydrochloric acid that aids digestions in the stomach.
Some industrial uses of sodium chloride include curing bacon, flavouring foods, and in the manufacture of margarine, butter and cheese. It is also used to tan leather in the leather industry. Rock salt is used as a fertilizer for sugar beet, and is spread on roads to melt the ice during winter. The salt is the starting point for many important chemicals, for example, the electrolysis of brine (concentrated solution of sodium chloride) gives sodium hydroxide, chlorine and hydrogen. What other uses of sodium chloride do you know? Mention them.
Calcium carbonate (marble, limestone, chalk)
Calcium carbonate finds a wide range of uses:
- An important use of calcium carbonate is in the building industry. It is widely used in making cement, lime, mortar and making steel from iron.
- Powdered limestone is used as a liming material to neutralize soil acidity. When used in this way, it is termed as agricultural lime. When added in the soil, agricultural lime acts as a calcium source for plants as well as increasing the pH and water retaining capacity of acidic soils.
- It is also used in making paint, plastic, rubber, ceramic and glass; and in oil refining, and iron ore purification.
- Calcium carbonate is the most preferred mineral in the paper industry. It helps in the production of the best quality papers.
- Since calcium is essential for healthy bones and teeth, it is used as a dietary calcium supplement.
- Limestone can also be well shaped, painted, and then used as decorative stones.
Ammonium salts
Most ammonium salts such as (NH4)3PO4, NH4Cl, NH4NO3, (NH4)2SO4, CAN, urea, etc are used as nitrogenous fertilizers which are applied to the soil to improve soil fertility and hence enhance plant growth and production. Millions of tonnes of fertilizers are produced every year. Without these chemicals, world food production would probably be halved.
Calcium sulphate (anhydride, CaSO4; gypsum, CaSO4.H2O)
Gypsum is chiefly used for the manufacture of Plaster of Paris (P.O.P). The Plaster of Paris is used for making casts for statuary ( the expansion during setting ensures a fine impression), in surgery to maintain joints in a fixed position and in cement and wall plasters.
Among the many other uses of calcium sulphate are as a pigment in white paints, as a soil conditioner, in Portland cement, as a sizer, filler, and coating agent in papers, in the manufacture of sulphuric acid and sulphur, in the metallurgy of zinc ores and as a drying agent in many laboratory and commercial processes.
Sodium carbonate (washing soda)
Sodium carbonate is widely used as one of the raw materials for making glass. Glass is made by heating a mixture of limestone, sand, sodium carbonate, and recycled glass in a furnace.The cosmetic industry uses it for manufacturing soap. The chemical industry uses it as a precursor to numerous sodium-containing reagents
It is also important in photography and in the textile industry. In addition to these industrial applications, sodium carbonate is used in medicine as an anti-acid
Sodium carbonate has various environmental applications. Large quantities of the carbonate are used in sewage treatment; in water softening as washing soda crystals, Na2CO3.10H2O, and in desulphurisation of flue gas.
Magnesium sulphate (Epsom salt)
Magnesium sulphate is mainly employed for making health salts (laxative, mild purgative). The health salt is used as a medicine (laxative) which aids to empty the bowels following constipation or other health problems.
Copper (II) sulphate
Copper (II) sulphate is used in fungicides, which are sprayed on crops, especially vines and potatoes, to kill moulds which would injure plants and hence curtail crop yield. It is also used in the manufacture of certain green pigments which are used for painting.
Calcium phosphate
TOPIC 4: THE MOLE CONCEPT AND RELATED CALCULATIONS.
The Mole as a Unit of Measurement
The Mole with Other Units of Measurements
Compare the mole with other units of measurements
When carrying out an experiment, a chemist cannot weigh out a single atom, ion, electron, proton or molecule of a substance. These particles are simply very small. A counting unit that is useful in practical chemistry must be used.
The standard unit is called one mole of the substance. One mole of each of these different substances contains the same number of the particles (atoms, molecules, ions, electrons, protons, neutrons, etc). That number per mole has been worked by several different experimental methods and is found to be 6.0 × 1023. The value 6.0 × 1023 is called Avogadro’s constant or Avogadro’s number and is abbreviated as L. It is named after the nineteenth-century Italian chemist, Amedeo Avogadro.
The value 6.0 × 1023 is obtained through the following relationship.The mass of one atom of carbon-12 is 1.993 × 10-23g. Then, the number of atoms present in 12g of carbon-12 is derived as follows:
1 atom = 1.993 × 10-23g
X atoms = 12g

\ X = 6.0 × 1023 atoms.
Therefore, the number of atoms in 12g of carbon-12 and hence the number of particles in a mole are 6.02 × 1023 atoms.
Hence, Avogadro’s number is the number of atoms in exactly 12g of carbon-12 isotope.One mole of any substance contains as many as many elementary particles as the Avogadro’s number (constant).
So, from the above explanation, the mole can be defined as the amount of a substance that contains as many elementary particles as the number of atoms present in 12g of carbon-12 isotope.
Substance Formula Relative formula mass, Mr Mass of one mole (molar mass) This mass (1 mole) contains
Carbon C 12 12g 6.0 × 1023 carbon atoms
Iron Fe 56 56g 6.0 × 1023 iron atoms
Hydrogen H2 2 × 1 = 2 2g 6.0 × 1023 molecules
Oxygen O2 2 × 16 = 32 32 6.0 × 1023 molecules
Water H2O (2×1) + 16 = 18 18g 6.0 × 1023 formula units
Magnesium oxide MgO 24 + 16 = 40 40g 6.0 × 1023 formula units
Calcium carbonate CaCO3 40+12+(3×16) = 100 100g 6.0 × 1023 formula units
Silicon oxide SiO2 28 + (2 × 16) = 60 60g 6.0 × 1023 formula units
Fe3+ Fe3+ 56 56g 6.0 × 1023 iron(III) ions
Cl- Cl- 35.5 35.5g 6.0 × 1023 chloride ions
e- e- - - 6.0 × 1023 electrons
The other substances, which also exist as molecules, include ozone molecule (gas), O3; phosphorus molecule (solid), P4; sulphur molecule, S8, etc.
In real life, when dealing with large numbers of small objects, it is usual to count them in groups. The objects are grouped and counted in unit amounts. For example, we buy a carton of soap, a gallon of kerosene, a crate of soda, a dozen of pencils, a ream of papers, etc.
Some units of measurement
Unit Number of objects per unit
Pair 1 pair = 2 objects, e.g. gloves, shoes, socks, scissors, etc are always sold in pairs.
Dozen 1 dozen = 12 objects e.g. a dozen of cups, plates, spoons, etc.
Gross 1 gross = 144 objects, e.g. a box of blackboard chalk contains 144 pieces of chalk.
Ream 1 ream = 500 objects, e.g. papers are sold in reams of 500 sheets.
Mole 1 mole = 6.02 ×1023 particles. In chemistry, extremely small particles are expressed in moles. For example:1 mole of atoms = 6.02 ×1023 atoms1 mole of electrons = 6.02 ×1023 electrons1 mole of protons = 6.02 ×1023 protons1 mole of ions = 6.02 ×1023 ions1 mole of molecules = 6.02 ×1023 molecules
Molar Quantities of Different Substances
Measure molar quantities of different substances
The mass of one mole of any substance (its molecular mass) is the atomic mass or molecular mass expressed in grams (or kilograms). For convenience, chemists prefer to express mass in grams, although the SI unit of mass is the kilogram. This is because the amount of substances which chemists usually work with in science laboratories, is quite small and if their masses are expressed in kilograms, the numbers used would be extremely small.
You can calculate the molar mass (M) of any substance by summing up the relative atomic weights of its constituents atoms. For example, ethanol, C2H5OH, contains two carbon atoms, six hydrogen atoms and one oxygen atom. So, the molar mass of ethanol can be calculated thus: Molar mass of C2H5OH = (2 × 12) + (6×1) + 16 = 46g.
In a similar way, molar masses of other compounds can be calculated. For example, the molar mass of sodium chloride, NaCl, is calculated by adding together the relative atomic masses of the constituents elements (Na = 23 and Cl = 35.5) = 23 + 35 = 58.5g (g mol-1).
It is important to note that relative atomic mass or relative molecular mass has no unit while molar masses are always expressed in grams or kilograms.
The molar mass of a compound is the same as the relative molecular mass and the molar mass of an element is the same as the relative atomic mass (Ar) of that element. The only difference lies in the units.
Example 1
M(CO2) = 44g (or g mol-1) = molar mass of carbon dioxide
Mr(CO2) = 44 = relative molecular mass of carbon dioxide
M(Fe) = 56g (or g mol-1) molar mass of iron
Mr(Fe) = 56 = Relative atomic mass of iron
Similarly, the molar masses of each of the following substances can be calculated using values for the relative atomic masses of the elements.
Molar masses of different substances
Substance Formula Molar mass
Ammonia NH3 14 + 1×3 = 17g
Ammonium chloride NH4Cl 14 + (1×4) + 35.5 = 53.5g
Lead (II) nitrate Pb(NO3)2 207 + (14×2) + (16×6) = 331g
Sulphuric acid H2SO4 (1×2) + 32 + (16×4) = 98g
Calcium carbonate CaCO3 40 + 12 + (16×3) = 100g
Potassium dichromate K2Cr2O7 (39×2) + (52 ×2) + (16×7) = 294g
Application of the Mole Concept
Known Masses of Elements, Molecules or Ions to Moles
Convert known masses of elements, molecules or ions to moles
In experimental work, chemists work with varying masses. They cannot always use one mole of a substance. The equation that links the mass of a substance to the number of moles present is:

Example 2
Convert 49g of sulphuric acid, H2SO4, into moles.Given:Mass = 49g; molar mass = 98g
Formula:

Solution:49g of H2SO4= 49/98= 0.5 mol.
Known Volumes of Gases at S.T.P to Moles
Convert known volumes of gases at S.T.P to moles
The volume occupied by one mole of a gas at standard condition of temperature and pressure has been scientifically determined, and it is found to be 22.4 dm3. This volume is called the molar volume of a gas. The molar volume of a gas, therefore, has the value of 22.4 dm3 at s.t.p. Remember that 1 dm3 (1 litre) = 1000 cm3. One important thing about this value is that it applies to all gases. Therefore, at s.t.p. 32g of oxygen (O2) or 17g of ammonia (NH3) or 44g of carbon dioxide (CO2) or 40g of argon (Ar) will occupy a volume of 22.4 dm3. This makes it easy to convert the volume of any gas at s.t.p. into moles, or moles into volume. However, it is important to note that as the conditions of temperature and pressurechange the molar volume will also change.
The number of moles of a given sample of gas is obtained by dividing the volume of the gas by molar volume (22.4 dm3).
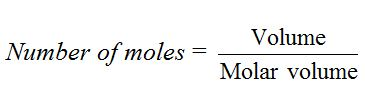
For example, 4.4d m3 of carbon dioxide gas at s.t.p. = 4.4/22.4= 0.196 mol.Similarly, 2.24 dm3 of neon gas at s.t.p. = 2.24/22.4= 0.1 mol.
If the volume of the gas is given in cm3, then it should be divided by the molar volume of a gas expressed in cm3. For example, 560 cm3of nitrogen gas = 560cm3/22400cm3 mol= 0.025 mol.
Alternatively, the volume may, first, be converted to dm3 and then divides by the molar volume, expressed in dm3, that is, 0.46dm3/22.4dm3 = 0.25mol
Masses of Solids or Volumes of Known Gases to Actual Number of Parties
Change masses of solids or volumes of known gases to actual number of parties
The number of particles in one mole of any substance is 6.02 × 1023. To find the number of particles in a substance, we use the expression:
- N = n.L, where
- N = the number of particles in that substance;
- n = the amount of substance (moles); and
- L = the Avogadro’s constant (6.02 × 1023).
This conversion requires two steps: first convert the mass of solid or volume of gas to moles, and then multiply the number of moles by the Avogadro’s constant. For example, to convert 5.6 dm3 of ammonia gas to the actual number of ammonia (NH3) molecules, change 5.6 dm3 of ammonia to moles =0.46dm3/22.4dm3=0.25 mol. Then multiply by the Avogadro’s constant to get the total number of molecules0.25 × 6.02 × 1023 = 1.5 × 1023 molecules
Similarly, 1.12 dm3 of hydrogen gas = 1.12/22.4= 0.05 mol. This is equal to 0.05 × 6.02 × 1023 = 3.0 ×1022 molecules
Alternatively, we may find out the number of particles by converting the given volume to the number of molecules straight forward without passing through the number of moles first. We know that one mole (22.4 dm3) of a gas at s.t.p. = 6.02 × 1023molecules. So, 5.6 dm3 = 5.6×6.02 × 1023/22.4= 1.5 × 1023 molecules
Molar Solutions of Various Soluble Substances
Prepare molar solutions of various soluble substances
A molar solution is a solution which contains one of the compound in one litre (1 dm3 or 1000 cm3) of the solution.Let us consider the case of sodium hydroxide, NaOH. The molecular weight of this compound is 40g. Therefore, a molar solution of sodium hydroxide will contain 40g in 1000 cm3(1 dm3) of the solution.
Also, consider anhydrous sodium carbonate, Na2CO3. 1 mole of this carbonate weights 106g. Hence, its molar solution will contain 106g of the anhydrous salt in 1000 cm3 of solution.If, however, 0.1 moles (10.6g) of the solute is dissolved in 1.0 dm3, the solution is 0.1 molar. But if 0.1 moles is dissolved in 0.1 dm3 of the solution, the solution is still 1.0 molar (since 1 dm3 of solution would contain 1.0 mole of the solute).
The molecular weights of some common substances are shown below:
| Compound | Molecular weight (1 mole) |
| Potassium hydroxide, NaOH | 56g |
| Hydrochloric acid, HCl | 36.5g |
| Sulphuric acid, H2SO4 | 98g |
| Sodium chloride, NaCl | 58.5g |
| Sodium bicarbonate, NaHCO3 | 84g |
| Calcium hydroxide, Ca(OH)2 | 74g |
The molar solution of each of these substances can be prepared by dissolving one mole of each substance in 1000 cm3 (1 dm3) of distilled water. We see, therefore, that 40g of sodium hydroxide in 1000 cm3 of solution will give a 1.0M solution. Hence, 20g of the hydroxide should give a 0.5M solution. In a similar way, we can make derivative solution concentrations ranging as follows: 0.1M, 0.2M, 0.3M, 0.4M….1M, 2M, etc.
However, in each case the amount of solution should always be 1000 cm3. The concentration ranges like these are known as molarities of solutions. Hence, 0.5M sodium carbonate can also be read as “a sodium carbonate solution with a molarity of 0.5M.”
The Concentration of Solutions
When a chemical substance (the solute) is dissolved in a given volume of solvent, we can measure the “quantity” of solute in two ways; we can measure either its mass (in grams) or its amount (in moles). The final volume of the solution is usually measured in dm3.
When we measure the mass of the solute in grams, we obtainthe mass concentration in g/dm3
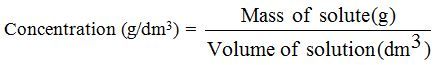
Example 3
Calculate the concentration (g/dm3) of sodium chloride solution (NaCl) that contains 20g of sodium chloride in a final solution of 100 cm3
Solution
First, convert the given volume to dm3
Volume (dm3) = 100/1000= 0.1 dm3
Then, work out the concentration of the solution by dividing the mass (weight) of solute (g) by the volume (dm3).
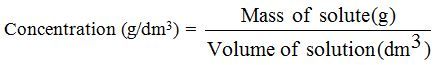
=20g/0.1dm3
= 200g/dm3
Alternatively, we could calculate the concentration straightforward without having to convert the given volume into dm3, e.g.:If 20g of the solution are contained in 100 cm3of the solution, then the amount of solute in 1000 cm3 (1 dm3) of the solution would be
1000×20/100 = 200g/dm3
Calculations Based on the Mole Concept
Perform calculations based on the mole concept
A chemist always wants to know how much of one substance would react with a given amount of another substance. This is achieved by use of balanced chemical equations. Such equations are called stoichiometric equations.
A stoichiometric equation is the one in which the reactants and the products are correctly balanced; all the atoms, ions and electrons are conserved. Such an equation gives correct mole ratios of reactants and products in chemical reactions. This quantitative relationship is called stoichiometry.
Consider an equation for the reaction between hydrogen and nitrogen to produce ammonia:
3H2(g) + N2(g)→ 2NH3
This can be read as follows:
three moles of hydrogen reacts with onemole of nitrogen to yield two moles of ammonia.
The numbers 3, 1 and 2 are called stoichiometric coefficients. They tell us the proportions in which the substances react and in which the products are formed.
Example 4
What volume of carbon dioxide (CO2) measured at s.t.p. will be produced when 21.0g of sodium hydrogencarbonate (NaHCO3) is completely decomposed according to the equation.2NaHCO3(s)→ Na2CO3(s) + CO2(g) + H2O(l)
Solution
First, find the weight of carbon dioxide that will be produced by the hydrogencarbonate.
- Mass of 2NaHCO3 = 2 × 84 = 168g
- Mass of CO2 = 44g
The weight of carbon dioxide produced can be obtained from the following relation:
168g ≡ 44g
21g ≡ X
X = 21×44/168 = 5.5g
The weight of carbon dioxide produced = 5.5g
Then, convert this weight of CO2 to volume at s.t.p.We know that one mole (44g) of carbon dioxide at s.t.p. occupies 22.4 dm3
That is, 44g ≡ 22.4dm3
5.5g ≡ X dm3
X = 5.5×22.4/44 = 2.8dm3
TOPIC 5: VOLUMETRIC ANALYSIS

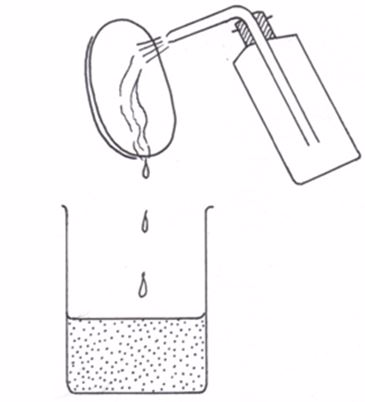
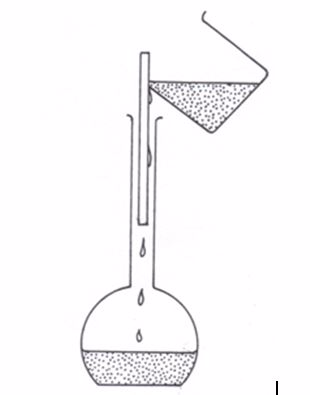
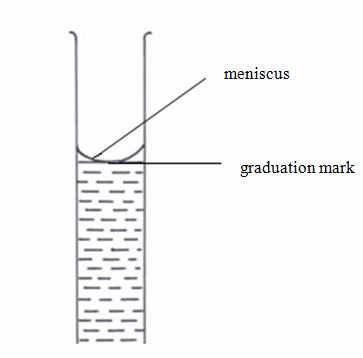
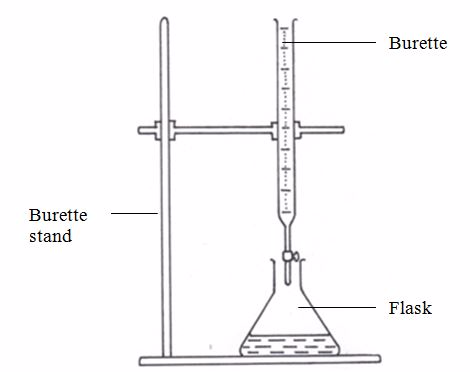
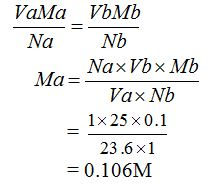
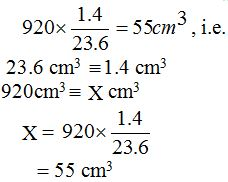
Standard Volumetric Apparatus
The Concept of Volumetric Analysis
Explain the concept of volumetric analysis
Volumetric analysis is a quantitative analysis involving the measurement of different solutions. These solutions are made to react completely and the completion of the reaction is indicated by certain substances called indicators. The quantitative composition of the solution is then determined.
Important steps of volumetric analysis include:
- Weighing;
- Preparation of the solution;
- Titration; and
- Calculation
In volumetric analysis, we deal with volumes of solutions. That is why this quantitative determination of solutions of substances is called volumetric analysis.
The amount of a substance present in a solution is given in terms of its volume and its concentration. The volume of a solution is usually given in litres (dm3). The concentration of a solution is given in moles per litre (mol/dm3) or grams per litre (g/dm3).
Volumetric analysis is a means of finding the concentration of an unknown solution. For example, the concentration of an unknown solution of an acid can be found if it is reacted with a standard solution of an alkali. A standard solution is one whose concentration is well known and does not change with time.
In volumetric analysis, the reaction is carried out in a carefully controlled way. The volumes are measured accurately using a pipette and burette. The method is to add a solution of one reactant to the solution of another reactant until the reaction is complete. When the reaction is complete, we say the end-point has been reached. If the reactants are acids and bases, completion (end-point) is determined by the change in colour of an acid-base indicator. The method is called titration. In other reactions, completion is determined by a colour change of reactant(s). The concentration of one of the reactant solutions must be known in order to be able to find the concentration of unknown solution.
Significance of Volumetric Analysis
- Volumetric analysis is used to quantify the amount of substances present in solutions by analytical procedure, which involves precise measurements of volumes of solutions and masses of solids.
- Volumetric analysis helps in the determination of the accurate volumes and concentrations of the reacting substances, often solutions.
- Volumetric analysis (titration) helps in the preparations of standard solutions.
- Volumetric analysis knowledge helps in the standardization of acids and bases.
Volumetric Apparatus
Use volumetric apparatus
We have seen that volumetric analysis involves determinations of quantities of substances, usually acids and alkalis, present in volumes of solutions. This is usually done by using measuring apparatus.
Apparatus used in volumetric analysis is based on volume measurements and since the analysis demands high accuracy, the apparatus has to be calibrated with the highest possible accuracy. It is for this reason that all apparatus for volumetric analysis are specifically for this and not other purposes.
Apparatus used for volumetric analysis include, burette, pipette, burette stand, white tile, conical flask, filter funnel, reagent bottle, watch glass, beaker, measuring cylinder and measuring flask (or volumetric flask). For approximate measurements, measuring cylinders may be used. For accurate measurements of volumes, volumetric flasks are used.
Burette
This is a long glass tube with a narrow lower part, which is fitted with a tap that controls the amount of solution let out of the burette. This instrument is calibrated from 0 to 50 cm3.Before measuring the solution, rinse the burette with distilled water, then with the solution it is going to hold. It has to be filled to the tip and all gas bubbles removed. Thus, the burette is an apparatus used for transferring the solution to the titration vessel (normally a flask).

Pipette
This apparatus has a wider middle part with narrow parts at either ends. The upper narrow part has a mark which marks the volume of all the space below it. If, say, the pipette is one that is marked 25 cm3, we can say that a solution, when filled in the pipette up to this mark, will have a volume of 25 cm3.
The pipette is used in transferring a standard solution to the titration flask. There are many types of pipettes depending on their volume capacity. The common ones are the 25-cm3and 20-cm3 capacity pipettes. Less common ones are the 10-cm3 capacity.Before measuring the solution, rinse the pipette several times with distilled water and then with the solution to be measured; suck the rinsing solution above the graduated mark, then discard the rinsing.

The pipette is commonly filled by mouth suction but the use of pipette fillers is highly recommended. When using a pipette, never blow out the last drop.

(a) A pipette (b) A pipette and pipette filler (used to fill and empty pipettes)
Measuring (Volumetric) flask
The flask is made of glass and has a mark at the upper part of the narrow tube. The space in the flask up to this mark represents a certain volume. If a solution is filled up to this mark, the volume of the solution is equal to the volume indicated by inscriptions on the flask e.g. 50 cm3, 100 cm3, 150 cm3, 250 cm3, 500 cm3, etc.

Filter funnel
A filter funnel is required for effective transfer of the weighed solid, liquid or solution into the volumetric flask or burette.

A filter funnel
Wash bottle
Wash bottle contains water and when squeezed, water squarts out. This is used in washing down the remains of the weighed solid into the volumetric flask.

A wash bottle
A weighing bottle
This is used in weighing the solute. It is a stoppered bottle. A watch glass can also be used to serve the same purpose.
Retort stand
A burette stand is used for holding the burette in place while carrying out volumetric analysis experiments.

A burette stand
Dropper
A dropper is used to add the indicator dropwise into the solution.

White tile or paper
A white tile or piece of paper is placed under the flask to give a clear background for accurate observation of the colour change at the end of the reaction (end point).

Standard Solutions
The Steps for Preparation of Standard Solutions of Common Acids
Explain the steps for preparation of standard solutions of common acids
A standard solution is a solution of known concentration. For example, a solution containing 15g of sulphuric acid in 1 dm3 of solution is a standard solution.
It has now been approved that volumetric work should be based upon the molar (M) solution. A 1 molar (1M) solution of a compound is a solution which contains one mole of that compound in 1 dm3 of the solution. For example, 58.5g of sodium chloride (NaCl) dissolved in 1 dm3 of the solution makes a molar solution of sodium chloride (1M NaCl). Likewise, 106g of sodium carbonate (Na2CO3) in 1 dm3 of the solution gives a molar solution of sodium carbonate. Therefore, a 1 molar sodium carbonate solution contains 106g of the salt in 1 dm3 of the solution.
1 molar solution of some compounds commonly used in titration contain the following masses of the compounds in 1 dm3of solution:
| Compound | Relative molecular weight (1 mole) |
| Sodium hydroxide, NaOH | 40g |
| Potassium hydroxide, KOH | 56g |
| Sulphuric acid, H2SO4 | 98g` |
| Hydrochloric acid, HCl | 36.5g |
| Sodium carbonate, Na2CO3 | 106g |
| Sodium bicarbonate, NaHCO3 | 84g |
Derivative concentrations are also used e.g. 0.1M, 0.5M. 2M, etc.
Preparation of standard solutions
A standard solution is required as a starting point for volumetric analysis. We learned early that in order to find the unknown concentration of a substance in volumetric analysis, the concentration of one of the solutions must be known.
A small range of substances are suitable for direct preparation of accurately standard solutions. Substances that cannot be used for direct preparation of standard solutions include sodium hydroxide, potassium hydroxide and concentrated sulphuric acid. These substances absorb water vapour from the air and hence cannot be weighed out precisely without taking extra precautions. Apart from absorbing water vapour from the air, sodium and potassium hydroxides react with carbon dioxide of the air to form respective carbonates.
2NaOH(s) + CO2(g)→ Na2CO3(s) + H2O(l)
2KOH(s) + CO2(g)→ K2CO3(s) + H2O(l)
Some solutions are volatile in nature and so are likely to change slowly in concentration during ordinary use. These include concentrated hydrochloric acid and ammonia.
A compound commonly used for preparation of a precisely standard solution is anhydrous sodium carbonate. It is best prepared from highly pure sodium carbonate. This is achieved by heating sodium bicarbonate to constant mass to make sure the compound is fully decomposed.
2NaHCO 3(s) →Na2CO3(s) + H2O(g) + CO2(g)
The sodium carbonate so formed is suitable for preparation of a standard solution and can be weighed without undergoing any appreciable change in composition.
Precautions to be observed while preparing a standard solution
- Transference of the substance from the weighing bottle to the beaker or flasks should be done with outmost care so that not a single particle of the substance is lost.
- Undissolved substance should not be transferred to the measuring flask. Make sure all the solid dissolves into solution before transferring the solution to flask.
- During making up of the volume, the last drop of the water should be added carefully. Do not blow out the final drop.
Standard Solutions of Bases
Prepare standard solutions of bases
Preparation of 0.1M sodium carbonate solution
The molecular mass of sodium carbonate (Na2CO3) is 106g. Therefore, a molar (1M) solution of sodium carbonate contains 106g in 1 dm3 (1000 cm3) of solution.
In order to prepare 0.1M solution of the carbonate, we have to weigh 10.6g of the carbonate and put it into a volumetric flask, which has a capacity of 1000 cm3.
However, normally 250 cm3 flasks are used. This means, in a 250 cm3 flask we have to add 10.6/4= 2.65g calcium of sodium carbonate.
Thus, 1000 cm3 ≡ 10.6g
250 cm3 ≡ X g
X = 250/1000×10.6 = 10.6/4 = 2.65g
The same procedure can be followed when preparing 0.25M, 0.5M, 2M, etc. of the solutions.
Procedure
- Weigh exactly 2.65g of sodium carbonate using a common balance and put it onto a watch glass.
- Transfer it slowly into a beaker of 500-cm3 capacity containing about 50 cm3 of hot distilled water.
- Wash down the watch glass with a jet of hot distilled water from a wash bottle and allow the washings to fall into the beaker (figure 5.7). Make sure all the sodium carbonate is washed into the beaker.
- Stir with a glass rod until all the solid is completely dissolved, and then cool the solution to room temperature. Leave the rod standing in the solution.
- Pour the solution carefully down the glass rod into a 250 cm3measuring flask.
- Wash the beaker out at least twice with jets of cold distilled water directed round the slides and pour the washings down the glass rod into the measuring flask (figure 5.8).
- Shake the flask gently and fill it up with cold distilled water almost to the mark.
- Add more distilled water drop by drop from a pipette until the meniscus is on the graduation mark (figure 5.9).
- Stopper the measuring flask and shake well. The liquid should then be exactly 0.1M sodium carbonate solution.
Preparation of standard solutions of other bases of different molarities e.g. 0.2M, 0.5M, 1.0M, 2.0M, etc. can be achieved by using the above procedures. The only variable will be the weight of the solids and volume of water as stated early.

Washing the watch glass

Filling the washings into the flask

Correct reading of the liquid volume
Acid-base Titration Experiments
Carry out acid-base titration experiments
Preparation of 0.1M sulphuric acid solution
A standard solution of sulphuric acid cannot be prepared directly because concentrated sulphuric acid is hygroscopic in nature (it tends to absorb water vapour from the air diluting itself) and is never reliably pure. A solution a little above 0.1M is prepared and then standardized and diluted with distilled water to exactly 0.1M.A molar solution of H2SO4 contains 98g of pure acid in 1 dm3. Therefore, the 0.1M acid contains 9.8g of the acid in 1 dm3 of the solution. The pure concentrated acid has a density (concentration) of about 1.8g/cm3. So, 9.8g of it occupy about 9.8/1.8 = 5.5cm3
The preparation of a standard solution of sulphuric acid involves two stages:
- Diluting a concentrated solution of the acid to an approximate molarity.
- Finding the exact concentration of the acid (standardizing it) by titrating it against a standard solution of a base (previously prepared).
Dilution of concentrated sulphuric acid
Caution: Make sure you wear safety goggles and gloves before carrying out this experiment.
Procedure
- Cautiously, because the acid is very corrosive, take 5.5 – 6.0 cm3of concentrated sulphuric acid in a small measuring cylinder.
- Pour the acid carefully, with stirring, into a 250-cm3 volumetric flask containing about 100 cm3 of cold distilled water.
- Pour this solution into, say, 700cm3 of cold distilled water in a measuring flask of capacity 1000cm3.
- Wash out the acid solution remaining in the measuring cylinder with cold distilled water twice and add the washings into the measuring flask.
- Then add distilled water approximately to the mark on the measuring flask, stopper it, and shake well.
This should give sulphuric acid of concentration a little above 0.1M. The diluted acid is now standardized with the 0.1M sodium carbonate solution prepared above.
Determination of the molarity (standardization) of sulphuric acid solution by titrating it against 0.1M sodium carbonate solution
The estimation of the concentration of a solution of an acid by reacting the acid with a standard alkali solution is known as titration. The end-point of an acid-base reaction is commonly determined by using a substance known as an indicator.
Procedure
- Measure 25 cm3 of 0.1M sodium carbonate solution and transfer it into a conical flask, using a pipette. Add a few drops of methyl orange indicator. This will turn the sodium carbonate solution yellow.
- Set up the apparatus as shown in figure 5.10
- Pour the acid into a 50-cm3burette. Read and note the level of the acid in the burette.

Titration setup
- By means of a tap at the base of the burette, drip the acid slowly into the conical flask, swirling the flask continuously until the colour of the liquid in the flask turns orange. This is the end-point of titration. Record the new level of acid in the burette.
- Repeat the titration three to four times, noting the initial and final reading of the burette each time.
- Find the volume of the acid as shown below:
Specimen readings
| Titration | Rough titration (Pilot) | Titre 1 | Titre 2 |
| Final burette reading | 24.20 | 23.65 | 23.55 |
| Initial burette reading | 0.00 | 0.00 | 0.00 |
| Volume of acid added | 24.20 | 23.65 | 23.55 |
Neglecting the first (rough) trial run, the average titration is 23.60 cm3
Calculation
The first step in calculating the molarity of any solution from the results of an acid-base titration is to write the equation for the reaction. From the equation, find the number of reacting moles of the acid and base.
Na2CO3(aq)1 mole+ H2SO4(aq)1mole→Na2SO4(aq) + CO2(aq) + H2O(l)
Now we have the following data.
- Volume of acid, Va = 23.60 cm3
- Volume of base, Vb = 25.00 cm3(this is the average amount of the base that was added to the flask in titration)
- Molarity of acid, Ma = ?
- Molarity of base, Mb = 0.1M
- Number of moles of acid, Na = 1
- Number of moles of base, Nb = 1
The molarity of the acid can be calculated from the following general formula:
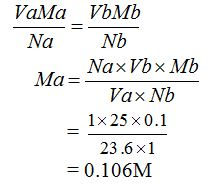
The concentration of sulphuric acid is 0.106M.
To make the molar concentration of the sulphuric acid solution exactly equal to that of the sodium carbonate solution (0.1M), 23.6 cm3of the acid must be diluted to 25 cm3, that is, 1.4 cm3of distilled water must be added to 23.6 cm3of the acid.
Remember it was stated early that in order to prepare 0.1M sulphuric acid solution, you need to dissolve 9.8g of the acid in 1000 cm3(1 dm3) of distilled water. Assume that some of the acid was wasted through spillage and mishandling and that only 920 cm3 of the acid was left. If, say, 920 cm3 of the acid was left, it can be made exactly 0.1M by the addition of 920×1.4/23.6 = 55cm3of distilled water. This gives exactly 0.1M of the acid. This is the same as saying that, if 23.6 cm3 of the acid were diluted with 1.4cm3 of distilled water, then 920cm3 of the acid would be diluted with
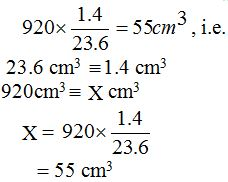
If, for instance, the volume of acid left was, say, 850 cm3, the amount of distilled water to be added would be = 850×1.4/23.6 = 50.4cm3. This, also, would give exactly 0.1M of the acid.
In principle, the amount of distilled water to be added is always calculated based on the amount of the acid left as exemplified above.
These two standard alkaline and acidic solutions can be used to standardize other solutions, e.g. sodium hydroxide, potassium hydroxide, hydrochloric acid, nitric acid, etc. You may dilute any base or commercial acid to some required concentration e.g. 0.2M, 0.5M, 0.25M, etc and then standardize it by similar procedures.
Choice of indicators in acid-base titration
We learned early that the estimation of the concentration of a solution of an acid or base by reaction with a standard alkali or acid solution respectively, is known as titration. The end-point of an acid-base titration is commonly determined using substances known as indicators, which usually portray certain characteristic colours when in alkaline or acid solutions.
The indicators in acid-base titrations must be chosen carefully because the choice of an inappropriate indicator would lead to an incorrect result. The choice of an indicator is based on the strength of an acid or base involved in the reaction.
There are three common indicators which are used in titration experiments involving acids and bases namely, methyl orange, litmus and phenolphthalein. The other indicators in less common use are as included in the table below. The table shows the colours which each of these indicators take up in acid or alkaline solution.
Colour of indicators in acid and alkaline solutions
| Indicator Colour of indicator |
| acid solution alkaline solution |
| Methyl orange pink yellow |
| Litmus red blue |
| Phenolphthalein colourless pink |
| Malachite green yellow blue/green |
| Thymol blue red yellow |
| Bromocresol green yellow blue |
| Bromothymol blue yellow blue |
Indicators suitable for particular types of acid-base reactions are as given in the table below:
Indicators suitable for different acid-base reactions
| Acid-base titration | Example | Choice of indicator | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Strong acid/strong base | H2SO4 and NaOH | Any indicator | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weak acid/strong base | CH3COOH (ethanoic acid) and KOH | Phenolphthalein | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Strong acid/weak base | HCl and NH3 | Methyl orange | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weak acid/weweakase | CH3COOH and NH3 | No satisfactory indicator available
Calculations
Common Mineral Acids
Standardize common minVolumetriceral acids
Data for calculations of volumetric analysis problems are obtained from volumetric analysis experiments. For any volumetric analysis problem, at least one standard solution is required. A correctly balanced reaction equation (from which moles ratios can be derived) is a prerequisite for all these calculations. This is because the mole ratio is an integral part of the general expressionused for all volumetric analysis calculations. The general expression is given by:
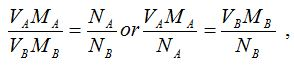
where;
The Relative Atomic Mass of Unknown Element in an Acid or Alkali
Find the relative atomic mass of unknown element in an acid or alkali
Example 1
12.5 cm3 of 0.5M sulphuric acid neutralized 50 cm3 of a given solution of sodium hydroxide. What is the molarity of the alkali?
Solution
Reaction equation is:
H2SO4(aq) + 2NaOH(aq) →Na2SO4(aq) + 2H2O(l)
From the equation, 1 mole of sulphuric acid solution reacts with 2 moles of sodium hydroxide solution. So, the number of moles of the acid, NA = 1 and the number of moles of base, NB = 2.The other data are as follows:
VA = 12.5 cm3, MA = 0.5M and VB = 50 cm3
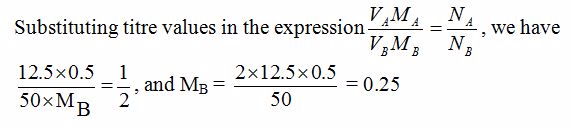
So, the molarity of the acid = 0.25M
Example 2
20 cm3 of a solution containing 7g/dm3 of a metal hydroxide, XOH, were exactly neutralized with 25 cm3 of 0.10M hydrochloric acid.
Solution
<!--[endif]-->HCl(aq) + XOH(aq)→ XCl(aq) + H2O(l)
From the reaction equation, NA = 1 and NB = 1.
VA = 25 cm3, VB = 20 cm3, MA = 0.1M, MB = ?
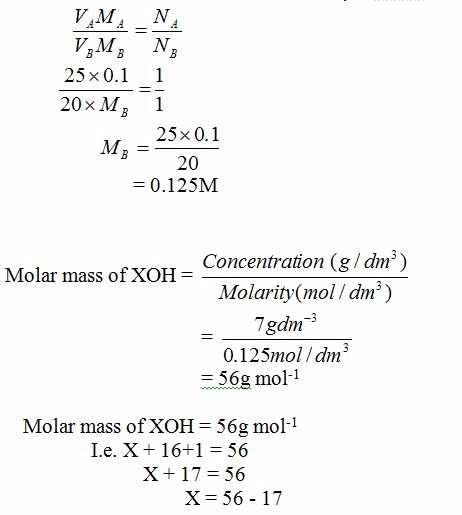
X = 39g
Therefore, element X is potassium (K)
The Percentage Purity of an Acid or an Alkali
Calculate the percentage purity of an acid or an alkali
Example 3
5.1g of impure sodium carbonate solution was dissolved in water to make 500 cm3 of solution. 20 cm3 of this solution was titrated against 20.45 cm3 of 0.04M hydrochloric acid. Calculate the percentage purity of the sodium carbonate solid.
Solution
To calculate the concentration of impureNa2CO3 solution:5.1g per 500 cm3 = 10.2g dm-3Reaction equation is:
Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
From the equation, NA =2 and NB = 1.
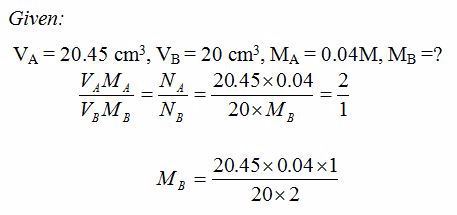
= 0.02045M
Therefore, molarity of the base = 0.02045M
To calculate the concentration of pure base (Na2CO3 solution) in g dm-3:
Concentration = Molarity × Molar mass
But molar mass of Na2CO3 = 106.
So, concentration (g/dm3) = 0.02045 × 106 = 2.1677g dm-3

The percentage purity of sodium carbonate = 21.25%
The Number of Molecules of Water of Crystallization of a Substance
Find the number of molecules of water of crystallization of a substance
Example 4
0.465g of a hydrated form of sodium carbonate exactly reacts with 75 cm3 of 0.10M hydrochloric acid. Calculate the number of molecules of water of crystallization present in one mole of the hydrated salt.
Solution
The balanced reaction equation for the anhydrous salt is:
Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l)
Number of moles of HCl present in 75 cm3 = 75/1000×0.1 = 0.0075M
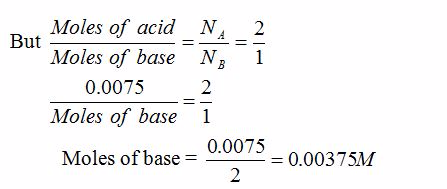
Mass of 0.00375 moles of Na2CO3 = 0.00375 × 106 = 0.3975g (= mass of anhydrous salt)
Molar mass of anhydrous salt = 106g
Mass of water contained in the hydrated salt = mass of hydrated salt - mass of anhydrous salt
= 0.465 – 0.3975
= 0.0675g
Therefore, the mass of water of crystallization = 0.0675g
But, remember that mass of the anhydrous salt = 0.3975g
Now, let the formula of the hydrated salt be Na2CO3.XH2O, where X is the number of moles of water crystallization
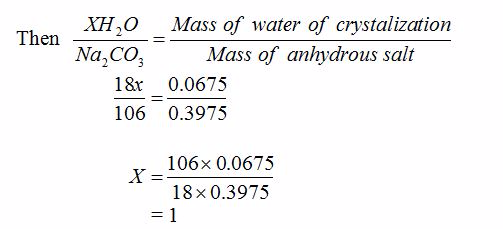
Therefore, the formula of the salt is Na2CO3.H2O
Application of Volumetric Analysis
The Application of Volumetric Analysis in Real Life Situations
Explain the application of volumetric analysis in real life situations
Volumetric analysis has a variety of laboratory and industrial applications in everyday life. The following are just a few of the applications (uses) of volumetric analysis in daily life:
Industrial and Laboratory Skills of Volumetric Analysis
Compare industrial and laboratory skills of volumetric analysis
The knowledge of volumetric analysis (titration) is used in hospitals and medical laboratories to carry out such duties as preparation of solutions and suspensions, blood analysis, and diagnosis of certain diseases and health problems. For example, when dissolving a solid drug to make a solution for injection, utmost precision is required to measure the correct volume of liquid to be used to dissolve a correct amount of solid drug to prepare the solution of a given concentration to inject to a patient.
Also titration is very important in the pharmaceutical industry, where precise measurements of quantities and concentrations are essential throughout the manufacturing process. Titration is thus an important part of the pharmaceutical industry to ensure quality control. Many variations of the titration technique are used, and specialized equipment for pharmaceutical titration is often developed to make the process more efficient.
TOPIC 6: IONIC THEORY AND ELECTROLYSIS
Ionic Theory
To account for the phenomena of electrolysis the Ionic Theory was put forward by Arrhenius in 1880. The theory states that electrolytes are made up of ions, which are built up in certain patterns called crystal lattice. When these substances dissolve in water, the structure is destroyed and the ions are set free to move.Concentrated mineral acids such as sulphuric acid, hydrochloric acid and nitric acid do not contain ions but they consist of molecules. However, when they are diluted, the molecular structure is destroyed and ions are formed.
Electrolytes and Non-electrolytes
Distinguish electrolytes from non-electrolytes
The main purpose of this chapter is to investigate the effects which electricity has on a range of substances, and to develop a thorough explanation of those effects in terms of our present knowledge of atomic structure. Before we begin, it is important that we familiarize ourselves with different terms that we are going to use to explain different phenomena. It is crucial that the definitions and meanings of these terms be understood at the outset in order that concepts defined in this chapter are easily and clearly apprehended. These terms are given hereunder:
Electrolytes and non-electrolytes
Liquids such as ethanol, paraffin, petrol and methylbenzene do not conduct electricity. The bonding in these compounds is covalent. These substances consist of molecules. There are no free electronsor charged particles to flow through them. Solutions of covalent compounds, for example sugar solution, do not conduct electricity.
These compounds are non-electrolytes. Non-electolytes exist only in the form of molecules and are incapable of ionization.
Ionic compounds contain charged particles (ions), but in solid state, the ions are firmly held in place and they are not free to move. An ionic solid does not conduct electricity. However, the ions present can become free to move if the solid is melted or dissolved in water. Then they can conduct electricity. For example, solid sodium chloride cannot conduct electricity but when melted or dissolved in water, the ions, Na+ and Cl- are set free. Then these ions are free to move in solution and hence conduct electricity. These compounds are called electrolytes.
Weak and Strong Electrolytes
Categorize weak and strong electrolytes
Weak electrolytes are compounds that are only partially or slightly ionized in aqueous solutions. Some substances, for example, ethanoic acid solution ionize partially.
CH3COOH(aq) ⇔CH3COO-(aq) + H+(aq)
Most of the electrolytes exist in solution in the form of unionized molecules. For example, in ordinary dilute (2M) ethanoic acid, out of every 1000 molecules present, only 4 are ionized and 996 are unionized.
A solution of ammonia water is also a weak electrolyte, containing a relatively small proportion of ammonium and hydroxyl ions.
NH4OH(aq) ⇔NH4+(aq) + OH-(aq)
Most of the organic acids are weak electrolytes, e.g. tartaric, citric and carbonic acids.
However, there is no sharp dividing line between weak and strong electrolytes.Water is also a weak electrolyte. It ionizes only slightly.
H2O(l)⇔H+(aq) + OH-(aq)
Study shows that for every molecule of water ionized, there are 6 million molecules of water not ionized.Strong electrolytes are compounds that are completely ionized in aqueous solutions. When sodium chloride is dissolved in adequate water it ionizes completely into Na+ and Cl- ions. There are no NaCl solid particles left unionized. All strong electrolytes (salts, the mineral acids and caustic alkalis) ionize completely in solutions.
The Mechanisms of Electrolysis
Electrolytic Cells of Different Electrolytes in the Molten and Aqueous States
Set up electrolytic cells of different electrolytes in the molten and aqueous states
The conductivity of ionic compounds is explained by the fact that ions move in a particular direction in an electric field. This can be shown in experiments with coloured salts. For example, copper (II) chromate (VI) (CuCrO4) dissolves in water to give a greensolution. This solution is placed in a U-tube. A colourless solution of dilute hydrochloric acid (HCl) is then layered on top of the salt solution in each arm. Graphite rods are fitted as shown in figure 13.3. These rods (electrodes) carry the current into and out of the solution.
After passing the current for a short time, the solution around the cathode becomes blue. Around the anode, the solution becomes yellow. These colours are produced by the movement (migration) of the ions in the salt. The positive copper ions (Cu2+) are blue in solution. They are attracted to the cathode (negative electrode). The negative chromate ions (CrO42-) are yellow in solution. They are attracted to the anode (the positive electrode). The use of coloured ions in solution has shown the direction that positive and negative ions move in an electric field. Always positive ions (cations) move to the cathode and negative ions (anions) move to the anode.
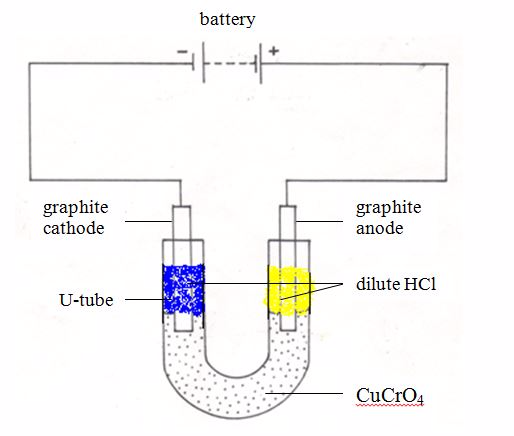
Ionic Migrations During Electrolysis and the Preferential Discharge of Ions at the Electrodes
Explain ionic migrations during electrolysis and the preferential discharge of ions at the electrodes
When a salt such as sodium chloride is dissolved in water, its ions are set free to move. So the solution can be electrolysed. Since the salts, alkalis and acids are dissolved in water, most of the solutions are aqueous. There is then a complication in electrolysis of such substances in aqueous form. This is because the water used to dissolve them also do ionize partially (it is a weak electrolyte)
Then at each electrode, we get more than one ion for discharge, but only one is supposed to be discharged. Take an example of electrolysis of copper (II) sulphate solution using platinum electrodes. By ionic theory, the solution ionizes thus:CuSO4 (aq) → Cu2+(aq) + SO42-(aq) (strong electrolyte)
During electrolysis, Cu2+ and H+ ions move to the cathode while SO42-and OH- ions move to the anode.
In situations like this, the order of discharge of the ions at the electrode will depend on:
The position of ion or radical in the electrochemical series
The arrangement of ions above is the same as that of the electrochemical series. If all other factors are constant, any ion will be discharged from solution in preference to those above it.
In other words, we can say that the reaction that occurs at the cathode is reduction(electron gain) and that which occurs at the anode is oxidation (electron loss).
Result
The concentration or nature of electrolyte
Take an example of electrolysis of sodium chloride using platinum electrodes when in:
aqueous solution
NaCl → Na+ + Cl-
H2O⇔H+ + OH-
During electrolysis:
Result
<!--[endif]-->Concentrated aqueous solution
NaCl → Na+ + Cl-
H2O⇔H+ + OH-
Results
Fused or molten sodium chloride
NaCl → Na+ + Cl-
During electrolysis:
Result
The nature of electrodes (inert vs active electrodes)
<!--[endif]-->If dilute sulphuric acid iselectrolysed using platinum electrodes:
H2SO4 → 2H+ + SO42-
H2O⇔H+ + OH-
Result
Charge flow during electrolysis
The coulomb is the electrolytic unit of charge. A current of one ampere is the rate of flow of charge equal to one coulomb per second.The charge is calculated from the knowledge of the number of seconds for which a steady current is passed.
Therefore charge = quantity of electricity (Q) = I×t
Flow of charge required to liberate 1 mole of element during electrolysis
Electrolysis always produces chemical reactions. Consider a reaction (at cathode) in which one mole of silver Ag+ ions is discharged and deposited.Ag+ + e- → Ag(s)
In this case, 1 mole of electrons (e-) is required to discharge 1 mole of Ag+ ions to produce 1 mole of silver atoms (Ag(s)). 1 mole of electrons is a large charge and experiments show that it is equal to 96500 coulombs. Therefore 1 mole of electrons = 96500 coulombs. This is called the faraday.
The number of faradays (moles of electrons) required to liberate 1 mole of an element during electrolysis is deduced from the equation for the electrode reaction.
Example 1
That is, 1 faraday is needed to deposit 1 mole of sodium atoms (23g), 3 faradays to deposit 1 mole of aluminium atoms (27g) and 2 faradays to liberate 1 mole of chlorine gas (71g).
Experiments to Identify the Products of Electrolysis when Different Electrolytes are Used
Perform experiments to identify the products of electrolysis when different electrolytes are used
Activity 1
Perform experiments to identify the products of electrolysis when different electrolytes are used
Experiments to Identify the Products of Electrolysis when Different Electrodes are Used
Perform experiments to identify the products of electrolysis when different electrodes are used
Activity 2
Perform experiments to identify the products of electrolysis when different electrodes are used
Laws of Electrolysis
Experiments to Relate Masses Liberated and Quantity of Electricity Passed
Carry out experiments to relate masses liberated and quantity of electricity passed
The laws expressing the quantitative results of electrolysis were first stated by a British chemist called Michael Faraday. The laws assert that the amount (expressed in moles) of an element liberated during electrolysis depends on:
An Experiment to Verify Faraday’s First Law of Electrolysis
Carry out an experiment to verify Faraday’s First Law of Electrolysis
The fact that the amount of a substance liberated during electrolysis depends upon these factors can be proved by conducting experiments. The product of time (measured in seconds) and the current passed (measured in amperes) gives a measure of electricity known as the quantity of electricity.
Quantity of electricity (Q) [coulombs]= current (I) [amperes]×time (t) [seconds]
Q = I × t
Because of this relationship, factors (1) and (2) may be included in the same experiment. The experiment to determine the effect of time on the amount of element deposited or liberated is carried out by passing a steady current through a solution of the compound of that element for different lengths of time.
The following table summarises the specimen results obtained by passing a steady current (0.21 amps) through a solution of copper (II) sulphate for 15, 30, 45 and 60 minutes. The last column shows the mass of copper deposited.
Specimen results for electrolysis of copper (II) sulphate
The relationship between the amount of copper deposited and the quantity of electricity passed can be assessed by considering the values in the last two columns in the table. This data may be represented in a graph illustrated in figure 6.4. The shape of the graph shows a straight line passing through the origin. From this fact, it is clear that the mass of copper deposited is directly proportional to the quantity of electricity passed. This is in accordance to what Faraday formulated in his First Law.
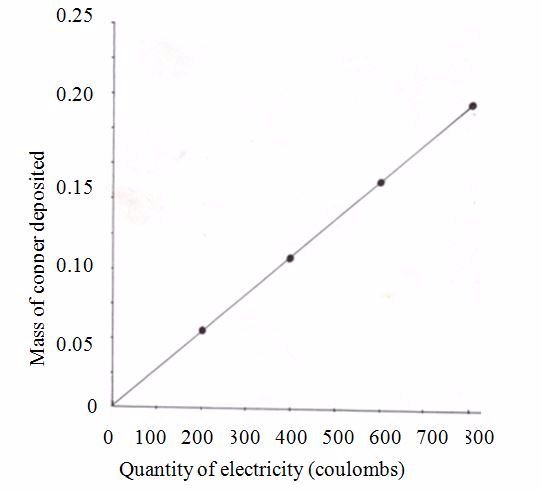
Graph of mass deposited versus amount of electricity passed
Faraday’s First Law of Electrolysis states that the mass of a substance liberated at (or dissolved from) an electrode during electrolysis is directly proportional to the quantity of electricity passing through the electrolyte.
The quantity of electricity is measured in coulombs where a coulomb is the passage of an electric current of one ampere for one second. Let
We can therefore represent the first law mathematically as:m α I × t or m = Z × I × t where Z is the proportionality constant referred to as electrochemical equivalent of the substance liberated. Electrochemical equivalent is the mass of a substance (element) liberated by 1 coulomb of electricity during electrolysis.
An Experiment to Verify Faraday’s Second Law of Electrolysis
Carry out an experiment to verify Faraday’s Second Law of Electrolysis
The third (3) factor mentioned previously as affecting the amount of substance liberated during electrolysis may also be investigated experimentally. Because our interest is the effect of the charge on the ions present in solution, we need to keep the quantity of electricity fixed whilst varying the types of the ions in solution. This may be achieved by passing the same quantity of electricity through two cells, with ions of different charges in each cell.
Apparatus for confirmation of Faraday’s Second Law
The experiment is conducted using two voltameters. The two voltameters are connected in series as shown. The first one is a copper voltameter and the second is a silver voltameter.The copper voltameter has copper electrodes in a solution of copper (II) sulphate. Hence, in this voltameter, copper ions are discharged and deposited at the cathode.
The silver voltammeter has silver electrodesin a solution of silver nitrate. The discharged silver ions are deposited at the cathode.
Before the experiment is started, each cathode electrode in the voltameters is cleaned, dried and weighed. The electrodes are then connected to the circuit, after which a suitable current is passed for a measured period of time.
After this, the cathode are removed from the voltameters, cleaned, dried and reweighed. The increase in mass of the two cathode electrodes represents the respective amounts of copper and silver deposited at the cathodes. The quantity of electricity required to deposit one mole of each element is calculated using Faraday’s Second Law of Electrolysis.
Specimen results
The results show that the masses of silver and copper deposited are different. A comparison of the amounts of each of the elements deposited can be made simple by calculating the number of moles of atoms of each of the element deposited.
Thus:
Amount of copper deposited = 0.221/63.5mole = 0.0035 mole
Amount of silver deposited = 0.755/107.8mole = 0.0070 mole
It is seen that twice as many atoms of silver are deposited as atoms of copper. The difference in amount of each element deposited arises from the difference in charges on ions of the element concerned.
The change on the copper ion is twice that on the silver ionand therefore twice the quantity of electricity will be required to liberate one mole of copper as for the liberation of one mole of silver.
This relationship is in accordance to Faraday’s Second Law of Electrolysis, which describes the relationship between the amount of element deposited and the charge on the ions of that element. Faraday’s Second Law of Electrolysis states that when the same quantity of electricity is passed through solutions of different electrolytes the relative numbers of moles of the elements deposited are inversely proportional to the charges on the ions of each of the elements respectively.
In order to discharge one mole of monovalent ions such as hydrogen ion, Sodium ion, Silver ion and Chlorine ion,96500 C of electricity are required. This quantity of electricity has been experimentally determined and is known as the Faraday constant.
It represents one mole of electrons, which is the same as the quantity of electrons required to discharge one mole of Siliverions to give one mole of silver atom. The validity of Faraday’s Second Law of Electrolysis is evident from the following observations:
Relationship between the Chemical Equivalents of Elements and Quantity of Electricity Passed
Relate the chemical equivalents of elements and quantity of electricity passed
A steady current of 4 amperes is passed through aqueous copper (II) sulphate solution for 1800 seconds using platinum electrodes. Calculate:
Given:
Solution
<!--[endif]-->Cathode reaction: Cu2+ + 2e- → Cu(s)
In this case, 2 Faradays of electricity are required to deposit one mole of copper atom 63.5g.
This means 2 × 96500 C liberates 63.5g of copper.
Quantity of electricity passed = I×t = 4×1800C. So, if 2×96500 C liberates 63.5g, thenI×t = 4×1800Cwill liberate2.4g of copper.
Therefore, mass of copper deposited = 2.4g
Anode reaction: 4OH-→2H2O(l) + O2(g) + 4e-
The reaction shows that 4 moles of electrons (4 Faradays) are lost during the reaction process.
Therefore, 4 × 96500 C are needed to liberate one mole (32g) of oxygen.
<!--[endif]-->Quantity of electricity flowing = 4 × 1800 C
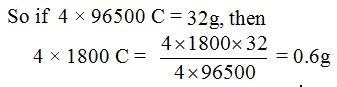
Therefore, mass of oxygen liberated = 0.6g
Application of Electrolysis
Electrolysis has several uses in industry. Its main application has been in the fields of manufacture of chemicals and in the purification of metals for which other purification methods prove either too difficult or highly expensive to apply. Some applications of electrolysis are as discussed below:
The Industrial Purification of Copper by Electrolysis
Outline the industrial purification of copper by electrolysis
Some metals can be purified by means of electrolysis. This process is used in industry to purify copper, which must be very pure 99.9% for electrical wiring. Copper made by roasting the sulphide ore is about 99.5% pure (so it has an impurity level of 0.5%). This level of impurity cuts down electrical conductivity significantly.
This is how the electrolytic purification (refining) process is carried out:The anode is made of a large block of impure copper. The cathode is a thin sheet of pure copper. The electrolyte is copper (II) sulphate solution.During the refining process, the copper atoms of the impure block become ions (the anode dissolves).Cu → Cu2+ + 2e-
The ions from the solution become atoms.
Cu2+ + 2e- → Cu(s)
They stick onto the cathode. A layer of pure copper builds up on the cathode. As electrolysis takes place, the cathode gains mass as copper is deposited on it. As a result, the cathode gets smaller while the cathode gets bigger as electrolysis proceeds. Eventually the whole cathode dissolves.
Purification of copper by electrolysis
Only pure copper sticks to the cathode. Most impurities fall to the bottom of the electrolytic cell. They form a solid material (anode sludge or slime) which contains small quantities of precious metals such as silver, gold and platinum. The precious metals recovered from the slime are purified and sold.
An Experiment on Electroplating of Metallic Materials
Carry out an experiment on electroplating of metallic materials
Electroplating is the coating of a metal with a layer of another metal by means of electrolysis. Electrolysis can be used to coat a thin layer of a less reactive metal onto a more reactive metal. The thin layer of less reactive metal will provide protection from corrosion for the more reactive metal underneath. It may also make the product more attractive.
The object to be coated should be made the cathode and the coating material should be the electrolyte. The most commonly used metals for electroplating are copper, chromium, silver and tin.
Steel can be electroplated with chromium or tin. This prevents the steel from rusting and gives it a shiny, silver finish. This is also the idea behind chromium-plating articles such as car bumpers, kettles, bath taps, etc. Chromium does not corrode, it is a hard metal that resists scratching and wear, and can also be polished to give an attractive finish.
Nickel can be electroplated with silver. This will make nickel more attractive.The diagram below shows how a steel jug is electroplated with silver. The jug becomes the cathode of an electrolytic cell. The anode is made of silver. The electrolyte is a solution of a silver compound, for example silver nitrate.
Silverplating a steel jug
At the anode: The silver dissolves, forming ions in solution:Ag → Ag+ + e-
At the cathode: The silver ions receive electrons, forming a coat of silver on the jug:Ag+ + e-→Ag (s)
When the layer of silver is thick enough, the jug is removed.In general, to electroplate any object with metal M, the set up is:
|




No comments